|
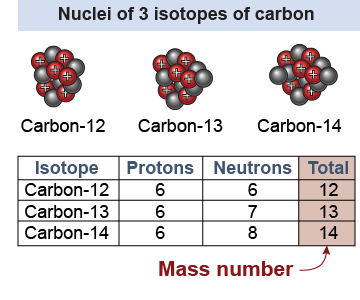 All atoms of the same element have the same number of protons, but they may have different numbers of neutrons. For example, a random sample of carbon atoms will contain several different isotopes. Isotopes are atoms that have the same number of protons but different numbers of neutrons. All the isotopes of carbon have six protons but one isotope has six neutrons, another has seven neutrons, and a third isotope has eight neutrons.
All atoms of the same element have the same number of protons, but they may have different numbers of neutrons. For example, a random sample of carbon atoms will contain several different isotopes. Isotopes are atoms that have the same number of protons but different numbers of neutrons. All the isotopes of carbon have six protons but one isotope has six neutrons, another has seven neutrons, and a third isotope has eight neutrons. 
|
The mass number is the total number of nucleons—protons plus neutrons—in the nucleus. Different isotopes are identified by their different mass numbers. For example, carbon-12 is the isotope of carbon with a mass number of 12, corresponding to six protons and six neutrons. Carbon-13 also has six protons but has seven neutrons for a mass number of 13. Equation (26.1) relates the atomic number Z to the mass number A and neutron number N. 
|
| (26.1) | | | A | = | atomic mass number
(number of nucleons) | | Z | = | atomic number (number of
protons or charge of nucleus) | | N | = | number of neutrons |
| Atomic mass number
|
|
Isotopes can be written with the full element name and mass number, such as carbon-13, or with the element symbol and a superscript preceding it to show the mass number, such as 13C. When physicists say “carbon twelve,” they are talking about the isotope of carbon with six neutrons and a mass number A = 12. 
|
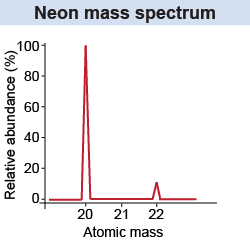 A random sample of most elements in nature will contain a mixture of isotopes. This is measured with a mass spectrometer, which you learned about on page 562. Its operation is based on the interaction of charge with electric and magnetic fields. For neon the detected signal has a large peak and a small peak. The large peak corresponds to A = 20 (20Ne) and the small peak corresponds to A = 22 (22Ne). The size of the peaks is directly related to the relative abundance of the isotopes neon-20 and neon-22, which is approximately 9:1.
A random sample of most elements in nature will contain a mixture of isotopes. This is measured with a mass spectrometer, which you learned about on page 562. Its operation is based on the interaction of charge with electric and magnetic fields. For neon the detected signal has a large peak and a small peak. The large peak corresponds to A = 20 (20Ne) and the small peak corresponds to A = 22 (22Ne). The size of the peaks is directly related to the relative abundance of the isotopes neon-20 and neon-22, which is approximately 9:1. 
|
Carbon-12 is a stable isotope, which means that an atom of 12C will remain 12C. Other isotopes may be unstable because they will spontaneously change into other isotopes. Carbon-14 is an example of an unstable isotope. Carbon-14 is radioactive and over time atoms of 14C decay into atoms of nitrogen-14. Radioactivity and the element carbon are discussed on page 802. 
|

