|
Lasers can be found in many everyday technologies, such as barcode scanners at the grocery store, laser pointers, and laser printers. They are also used in metal cutting, communications, medical surgery, and astrophysics. Have you ever wondered how they work? 
 |
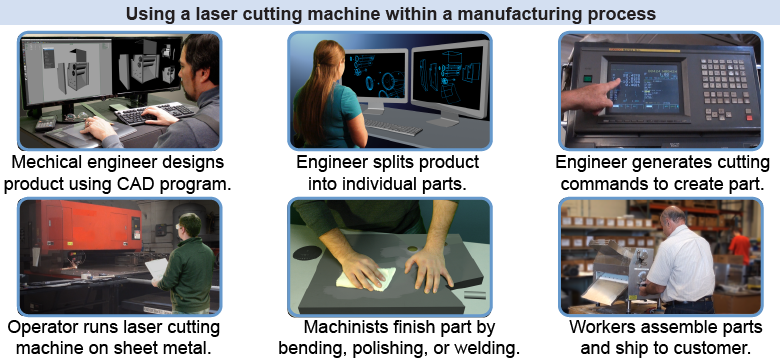 A laser can be used for cutting sheet metal in a factory to make parts for a product—such as a restaurant machine to press dough flat for a pizza. How might this laser cutting tool be used within the manufacturing process?
A laser can be used for cutting sheet metal in a factory to make parts for a product—such as a restaurant machine to press dough flat for a pizza. How might this laser cutting tool be used within the manufacturing process? - A mechanical engineer designs the product using a computer-aided design (CAD) program.
- The engineer separates the complete product into individual parts. Some parts need to be machined, while others (such as electrical cables) might be “off-the-shelf.”
- For each machined part, the engineer generates a series of cutting commands for the laser machine—such as “move over 5 cm and cut for 2.3 cm” or “cut a series of holes spaced 1 cm apart.”
- The machine operator uploads the computerized commands to the cutting machine’s control computer, loads a sheet of metal, and runs the machine.
- A machinist takes the cut part and uses various machining tools to smooth sharp edges, fold the metal where needed, insert pins, weld seams together, and so on.
- Workers assemble various pieces together into the product and test it before shipping the product to a customer.
Some of the advantages of using a laser cutting machine within this manufacturing process are that it can create smaller holes, cut thicker sheets of metal, cut faster than a machinist working alone, and can run automated overnight without stopping! 
|
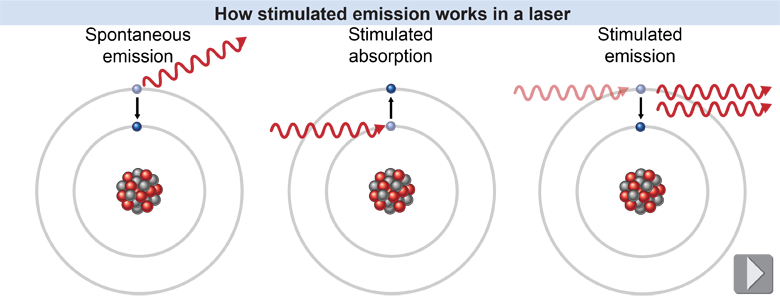
The laser is based on atomic energy level transitions—but with a twist. When an electron transitions on its own from a higher to a lower energy level, this is called spontaneous emission, and the atom loses energy in the form of a photon. It is called spontaneous because it is not caused by an external force or energy. The emitted photon can travel in any direction. For the electron to transition from a lower energy to a higher energy level, however, it must gain energy from an external source—namely, by the stimulated absorption of a photon. Stimulated absorption is another name for the absorption of a photon. Spontaneous emission and stimulated absorption are the two kinds of energy level transitions that were covered earlier in this chapter. The change in energy between the two atomic levels corresponds to the energy of the photon, whether it was absorbed or emitted. 
|
|
In the laser, a different kind of emission of a photon takes place. In stimulated emission an incoming photon induces the atom to change from a higher to a lower energy state. For this to happen, the incoming photon must have an energy exactly equal to the difference between the two energy levels for the atom. There are five key features of stimulated emission: - Energy is required initially to raise the electrons to a higher energy level.
- Both the energy and the direction of the incoming photon are not affected.
- The emitted photon travels in exactly the same direction as the incoming photon.
- The emitted photon is exactly in phase with the incoming photon.
- The incoming light is amplified, because for every one input photon there are two output photons.
The laser is based on this physical process of stimulated emission. The term laser is an acronym for light amplification by stimulated emission of radiation, coined in 1957 by physicist Gordon Gould of Columbia University. 
|
In stimulated emission, where is the electron in the atom before it interacts with the photon: at the higher energy level or at the lower energy level?
 |
The electron is at the higher energy level, so when it gets hit by the photon, it will drop down and release its energy. It is raised up to that level by energy from some other source, such as a battery. 
|
| |
|

