|
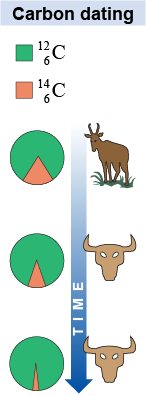 Carbon dating is the technique of comparing the concentrations of carbon-12 and carbon-14 to determine the age of a biological material. While the most common isotopes of carbon, and , have stable nuclei, is radioactive. Carbon-14 is produced steadily in Earth’s upper atmosphere, where nuclei interact with neutrons produced by cosmic rays entering the atmosphere. Living organisms constantly exchange carbon with the environment maintaining a constant ratio of about one atom of carbon-14 for every 1013 atoms of carbon-12. When an organism dies, active carbon exchange with the environment stops. Carbon-14 already within the organism decays radioactively with a half-life of 5,730 years. This decay provides a natural clock which starts ticking the moment an organism dies. For example, carbon dating can determine the age of ancient Egyptian papyrus samples and the charcoal from prehistoric campfires. Carbon dating works reliably up to about 10 times the half-life, or 57,300 years. After 10 half-lives there is not enough carbon-14 remaining to measure accurately.
Carbon dating is the technique of comparing the concentrations of carbon-12 and carbon-14 to determine the age of a biological material. While the most common isotopes of carbon, and , have stable nuclei, is radioactive. Carbon-14 is produced steadily in Earth’s upper atmosphere, where nuclei interact with neutrons produced by cosmic rays entering the atmosphere. Living organisms constantly exchange carbon with the environment maintaining a constant ratio of about one atom of carbon-14 for every 1013 atoms of carbon-12. When an organism dies, active carbon exchange with the environment stops. Carbon-14 already within the organism decays radioactively with a half-life of 5,730 years. This decay provides a natural clock which starts ticking the moment an organism dies. For example, carbon dating can determine the age of ancient Egyptian papyrus samples and the charcoal from prehistoric campfires. Carbon dating works reliably up to about 10 times the half-life, or 57,300 years. After 10 half-lives there is not enough carbon-14 remaining to measure accurately. 
|
The rate of radioactive decay—how many nuclei decay per unit time—is related to the half-life t½. If we have a radioactive sample that initially has N0 nuclei, then after time t the number of radioactive nuclei remaining in the sample is given by equation (27.2). 
|
| (27.2) | | | N | = | amount of sample after time t | | N0 | = | initial amount of sample | | t | = | time elapsed (s) | | t½ | = | half-life (s) |
| Decay rate equation
|
 |
How can we calculate the amount of time that has elapsed to produce a particular concentration of a radioactive isotope? If we know t½, N0, and N, then we can solve for time t. The equation above relates the current concentration to initial concentration and half-life. Since time is in the exponent, we start by taking the logarithm of both sides of the equation: | |
Now solve for the time t: | |

|
The general technique for dating matter using radioactivity is called radiometric dating. Besides carbon-14, a number of other radioactive isotopes are used for dating. For example, climate scientists measure the concentration of oxygen-18 and oxygen-16 in ice core samples to understand the composition of the atmosphere over time. Geologists use this same technique to date rock samples by measuring the concentration ratio of uranium-238 to plutonium-239. This technique allows geologists to explore geologic times because the half-life of uranium-238 is 4.5 billion years. 
|
An ancient fern specimen has one-eighth the amount of carbon-14 per gram as compared to a living creature. How old is the specimen? | Asked: | the number of years ago the fern died | | Given: | The mass of carbon-14 has decayed to one-eighth its original value. | | Relationships: | For the time period of one half-life, half of the radioactive atoms will decay radioactively.
The half-life of carbon-14 is 5,730 years. | | Solution: | After one half-life, half of the carbon-14 atoms will be left. After the second half-life, one-half of the remaining one-half carbon-14 atoms will remain, or one-fourth. After the third half-life, one-eighth will remain. The fern therefore died three half-lives of carbon-14 ago: 3 × 5,730 years is 17,190 years. | | Answer: | The fern died 17,190 years ago. | 
|
| |
|

