|
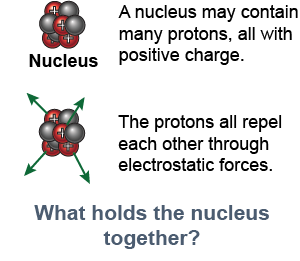 The nucleus contains both protons and neutrons. Since protons are positively charged and neutrons do not have any charge, why does the nucleus stay together? The electrostatic repulsion between two protons is enormously strong and should tear the nucleus apart instantly. The answer is that there is another type of force that is attractive and even larger than the electrostatic force. This attractive force is what keeps the nucleus together and it is called the strong nuclear force.
The nucleus contains both protons and neutrons. Since protons are positively charged and neutrons do not have any charge, why does the nucleus stay together? The electrostatic repulsion between two protons is enormously strong and should tear the nucleus apart instantly. The answer is that there is another type of force that is attractive and even larger than the electrostatic force. This attractive force is what keeps the nucleus together and it is called the strong nuclear force. 
|
|
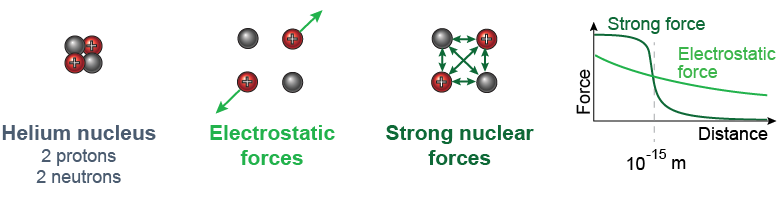
The strong nuclear force attracts every proton or neutron to every other proton or neutron regardless of electric charge. The strong force binds together the protons and neutrons in the nucleus with such a great attractive force that enormous amounts of energy are involved in any process that changes the nucleus. 
|
The strong force is peculiar in that it falls off in strength very quickly at distances greater than 10−15 m. For this reason the strong nuclear force is called a short-range force. While the strong nuclear force is very powerful within the nucleus, its short-range nature means it is much weaker than the electrostatic force over the greater volume of an atom outside the nucleus. 
|
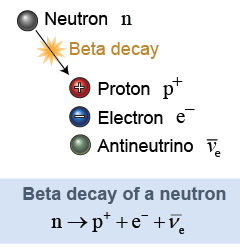 Physicists know of four fundamental forces in nature that govern the behavior of everything from the motion of planets to the motion of atoms. These forces are gravity, electromagnetism, the strong nuclear force, and the weak nuclear force. The strong force affects protons and neutrons but has no effect on electrons. The weak nuclear force affects electrons and other particles called neutrinos. Neutrinos are very light, almost massless particles similar in some ways to photons of light. The weak force causes a free neutron outside a nucleus to break up spontaneously into a proton, an electron, and a type of neutrino. This process, called beta decay, is one form of radioactivity discussed further in Chapter 27.
Physicists know of four fundamental forces in nature that govern the behavior of everything from the motion of planets to the motion of atoms. These forces are gravity, electromagnetism, the strong nuclear force, and the weak nuclear force. The strong force affects protons and neutrons but has no effect on electrons. The weak nuclear force affects electrons and other particles called neutrinos. Neutrinos are very light, almost massless particles similar in some ways to photons of light. The weak force causes a free neutron outside a nucleus to break up spontaneously into a proton, an electron, and a type of neutrino. This process, called beta decay, is one form of radioactivity discussed further in Chapter 27. 
 |
The beta decay of a neutron produces an electron antineutrino. There are three kinds of neutrinos known: electron neutrinos, muon neutrinos, and tau neutrinos. Each of these has an antimatter partner with opposite quantum properties. One of the properties is called the lepton number. An electron has a lepton number of +1. A neutrino also has a lepton number of +1. An antineutrino has a lepton number of −1. We observe that the total lepton number is conserved in nuclear reactions, such as beta decay. The neutron has a lepton number of zero, as does the proton. Since the single neutron starts with a lepton number of zero the total lepton number after the reaction must also be zero. That is why an antineutrino is produced, to cancel the lepton number of the electron. 
|
| |
|

