|
How do electrons “know” which orbits to occupy in an atom? In his 1913 paper, Bohr showed that this behavior would explain the spectrum but he could not say why the electron could occupy only those orbits. Ten years later in 1924, French physicist Louis de Broglie made a most unusual proposal. He proposed that there was an intrinsic connection between waves and electrons. When electrons were confined to a small space, de Broglie suggested that they have a wavelength related to energy, like light. 
|
|
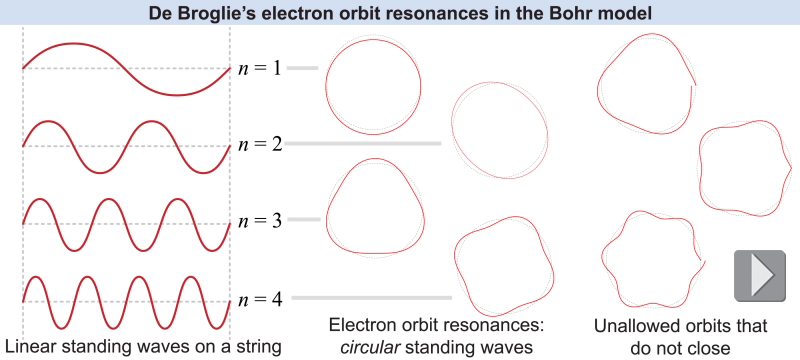
If electrons had a wavelength then certain orbits would be resonances of the electron’s “wavelength” as it traveled around the circumference of its orbit. This is similar to the harmonics on a vibrating string. Bohr’s fixed orbits would correspond to standing waves, occurring when one complete electron wavelength exactly matched the circumference of a circular orbit. Since the circumference of a circular orbit depends on its radius, restricting the circumferences to integer multiples of wavelength forced the electron to be at specific distances from the nucleus. This explained why the electron orbits have discrete energies. The smallest orbit would be the fundamental, the next would be the second harmonic, and so on. Electrons would not be found at any other wavelengths because the electron “wave” would undergo destructive interference with itself. 
|
The radius of the electron’s orbit in the Bohr model is at integer multiples n = 1, 2 , 3, ... of the minimum radius. These integer values of n are called the quantum number for the electron’s orbit. 
|
One of the results of quantum theory is that, when a particle is confined to a small system, such as within an atom, the energy the particle can have is quantized. The term quantized means that the particle’s energy can only assume discrete values determined by the system. An electron in a quantum system may have an energy corresponding to the first quantum level, or the second level, or the third. The electron cannot, however, have an energy in between the quantized values. A remarkable insight of Bohr’s model of the hydrogen atom is how closely the quantum theories of light and the atom are linked. The atom can change its energy through emission and absorption of single photons of light. 
|
How does the wave behavior of an electron explain quantized orbits?
 |
The electron creates a standing wave around its orbit. At orbits of different radii, a different number of wavelengths fit in. The electron can only occupy orbits where an integer number—no fractions—of wavelengths fit. 
|
| |
|

