|
When an atom makes a transition to a lower energy level, where does the excess energy go? Likewise, where does an atom get the energy needed to make a transition to a higher energy level? The answer to both questions involves light. Atoms change their internal energy by rearranging electrons among energy levels through emitting or absorbing light. 
|
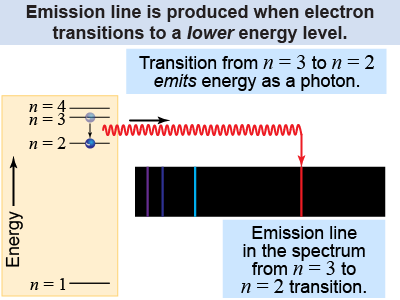
| If an atom lowers its energy by ΔE, a photon is emitted with a frequency given by Planck’s relation ΔE = hf. Discrete spectra occur because the atom’s energy is “stored” in the arrangement of its electrons. The atom can only lose an amount of energy corresponding to the difference between two energy levels. This restricts the frequency and wavelength of light emitted. For example, a hydrogen atom that drops an electron from the n = 3 energy level to n = 2 gives off red light with a wavelength of 656 nm, corresponding to the red line in the hydrogen spectrum. |

|
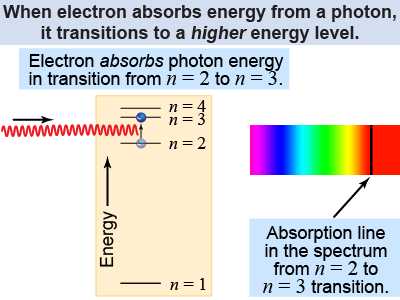 An atom gains energy when it absorbs a photon of light. Absorption is also quantized and an atom can only absorb light with a photon energy equal to the difference between two energy levels. For an atom to increase its energy by ΔE it must absorb a photon with a frequency given by ΔE = hf. For example, a hydrogen atom absorbs a photon of red light with a wavelength of 656 nm by promoting one electron in the n = 2 energy level up to the n = 3 level.
An atom gains energy when it absorbs a photon of light. Absorption is also quantized and an atom can only absorb light with a photon energy equal to the difference between two energy levels. For an atom to increase its energy by ΔE it must absorb a photon with a frequency given by ΔE = hf. For example, a hydrogen atom absorbs a photon of red light with a wavelength of 656 nm by promoting one electron in the n = 2 energy level up to the n = 3 level. 
|
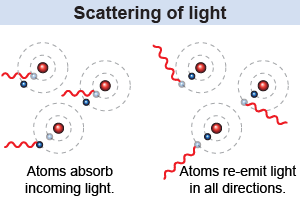 Imagine that an atom absorbs a photon of light to raise its energy level, but soon after it emits another photon of light to lower its energy level back again. In what direction does the newly emitted photon travel? The answer is that the photon may be emitted in any direction. When an atom emits a photon of light, there is no telling in advance what direction it will be traveling. This curious property of the absorption and re-emission of light by atoms is called scattering. You can see scattering of light every day in the blue sky and red sunsets.
Imagine that an atom absorbs a photon of light to raise its energy level, but soon after it emits another photon of light to lower its energy level back again. In what direction does the newly emitted photon travel? The answer is that the photon may be emitted in any direction. When an atom emits a photon of light, there is no telling in advance what direction it will be traveling. This curious property of the absorption and re-emission of light by atoms is called scattering. You can see scattering of light every day in the blue sky and red sunsets. 
 |
If particles in the Earth’s atmosphere are scattering light, then why don’t we see all different colors scattered, not just blue? The answer is that the kind of scattering going on in the Earth’s atmosphere is strongly dependent on the wavelength of the light owing to the size of the particles scattering the light. In effect, longer wavelengths of light can pass easily around small particles in the atmosphere, whereas shorter wavelengths of light scatter off of the particles. This is analogous to waves encountering a rock: Long waves easily pass around a small stone, but ripples will encounter diffraction or scattering. Blue wavelengths of light are shorter than red wavelengths, so blue light scatters more easily than red light. That is why we see blue scattered light all over the daytime sky. 
|
When an electron in a hydrogen atom transitions from the n = 3 energy level to n = 2 it loses 1.89 eV (3.03×10−19 J) of energy. What is the frequency of light emitted during this process? - 3.51×10−34 Hz
- 2.19×10−15 Hz
- 4.57×1014 Hz
- 2.85×1033 Hz
 |
The correct answer is c.
Asked: frequency f of emitted electromagnetic wave
Given: change in energy, ΔE = 3.03×10−19 J
Relationships: ΔE = hf, Planck’s constant h = 6.63×10−34 J s
Solve: Rearrange and solve the Planck relation for f: 
|

