|
Newton’s laws along with concepts of force, velocity, and acceleration were adequate to describe everything humans knew up until around the turn of the 20th century. The experiments that revealed the structure of the atom cast classical physics on its head because we observed many things that classical physics could not explain. Between 1900 and 1925 a new branch of physics, quantum physics, took shape and our understanding of nature has never been the same. Quantum physics describes the physical laws at a small scale—the scale of the atom and the elementary particles. At the microscopic scale, particles such as electrons do not move according to Newton’s laws. Light, which has no mass, is found to have momentum. Even though we do not directly perceive the quantum world, many technologies, from GPS satellites to lasers, are derived from quantum physics. 
|
Wave–particle duality
|
The quantum theory of light is very different from the wave theory. Wave theory says that you can reduce the energy of a light wave as much as you want by reducing the amplitude. According to quantum theory, however, you cannot split a photon. Light can be 1 photon, 10 photons, or 10 trillion photons—but never half a photon. As we learned on page 649, Planck discovered that light has a particle nature when observed on a very small scale. 
|
| (26.2) | | | E | = | energy (J) | | h | = | Planck’s constant = 6.63×10−34 J s | | f | = | frequency (Hz) |
| Photon energy
|
|
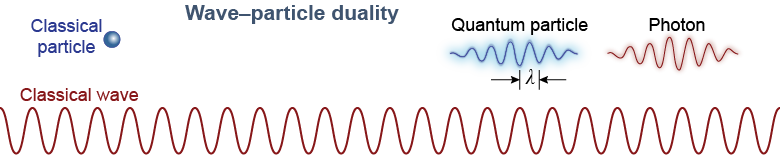
A classical particle is a like a tiny ball. It has a definite size, mass, and position. In 1924, Louis de Broglie proposed that this intuition is wrong when things are as small as an atom. In the quantum world, a particle is not like a tiny ball at all. Instead, its mass, size, and even its location is spread out into a wave. The wavelength of de Broglie’s matter wave is given by equation (26.3). 
|
| (26.3) | | | λ | = | wavelength (m) | | h | = | Planck’s constant = 6.63×10−34 J s | | p | = | momentum (kg m/s) |
| de Broglie wavelength
|
|
|
The fact that light has particle aspects and matter has wave aspects on the quantum level is called wave–particle duality, which we learned about on page 653. The particle nature of light becomes evident when the energy of a system gets close to the photon energy. The wave nature of matter becomes evident when the size of a system becomes comparable to the de Broglie wavelength. Both equations involve Planck’s constant h, which is a characteristic of the quantum world. 
|
| |
|

