| | Essential questions | | How do we know that the atom contains a small, positively charged
nucleus
containing nearly all the atom’s mass? | |
|
How did Ernest Rutherford determine that the nucleus of the atom was small, massive, and positively charged? In this interactive simulation, you will bombard a nucleus with alpha particles and watch their trajectories. You will have the opportunity to expand on Rutherford’s experiment by using different masses of target nuclei. 
|
Background on Rutherford scattering
Rutherford, in his famous experiment, shot alpha (α) particles at a thin foil of gold atoms as a way of probing the internal structure of the atom. An alpha particle is the nucleus of a helium atom, consisting of two protons and two neutrons. Rutherford observed that most of the alpha particles passed through the foil unaffected. But some of the alpha particles were deflected by the gold atoms, which he interpreted as evidence for a dense, massive, and positively charged nucleus of the atom. How did his experiment work? 
|
Simulation of Rutherford scattering
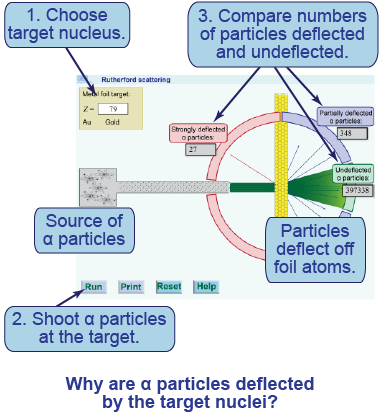
- Choose gold (Z = 79) as the target nucleus.
- Press play to shoot α particles at the target nuclei of the gold foil. Allow the simulation to run for some time to collect sufficient numbers of deflected particles.
- Compare the number of undeflected particles to the number deflected both partially and strongly by the target atoms.
- What fraction of alpha particles are deflected by a gold nucleus? Provide evidence to support your conclusion.
- What do you think happens to alpha particles that pass near the nucleus? Those that pass far from the nucleus? Why?
- Change the number of protons in the target nucleus. How do the trajectories of the alpha particles change? Why?
- Use the evidence from this simulation to argue that the nucleus of the atom must be (i) small, (ii) massive, and (iii) positively charged.

|
|
In this interactive simulation, you will shoot alpha particles at a thin foil of gold atoms. Which alpha particles are deflected? Why? What do these results indicate about the structure of the atom?
|
| |
|

