|
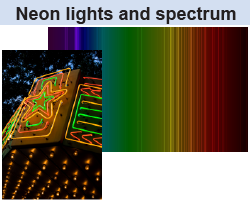 When electricity is passed through a low-pressure neon gas inside a glass tube, the gas glows. Neon signs are usually red, although they can also have green, yellow, or orange colors. But they are never blue! Why? Neon gas has unique emission lines in the green, yellow, orange, and red wavelengths of visible light but none at blue wavelengths. If you ever see a glowing blue sign at night you will know one thing: It’s not a neon sign. Some other gas is inside the tube!
When electricity is passed through a low-pressure neon gas inside a glass tube, the gas glows. Neon signs are usually red, although they can also have green, yellow, or orange colors. But they are never blue! Why? Neon gas has unique emission lines in the green, yellow, orange, and red wavelengths of visible light but none at blue wavelengths. If you ever see a glowing blue sign at night you will know one thing: It’s not a neon sign. Some other gas is inside the tube! 
|
Spectrum of hydrogen and the Bohr atom
|
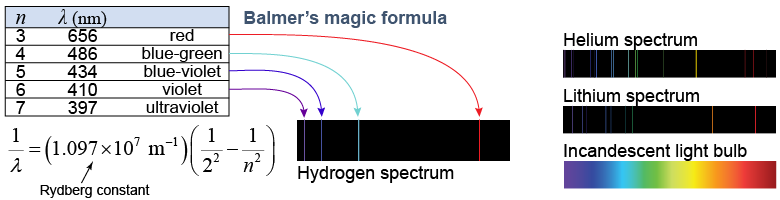
When electricity is passed through hydrogen gas, the gas glows and gives off light. The light, however, is not like the light given off by an incandescent light bulb. Instead of a smooth rainbow of colors, hydrogen gas gives off a few very specific colors and nothing in between. Other elements were observed to give off different patterns of colors. The pattern, called a spectrum, is unique for each element. Why this occurred was a mystery. In 1885, a Swiss school teacher named Johann Balmer discovered that the wavelengths of the light in the hydrogen spectrum obeyed a precise mathematical relationship, now known as Balmer’s formula. 
|
|
Balmer had found the pattern but he could not explain why it occurred. In Balmer’s formula, n is an integer, such as 3, 4, or 5. When n = 3, the formula predicts a wavelength of 656 nm, which matches exactly the red line in the hydrogen spectrum. What the formula implied was that something inside hydrogen atoms acted like a series of switches. The atomic switches could be set to any integer, such as 3 or 4, but not any number in between, such as 2.5. 
|
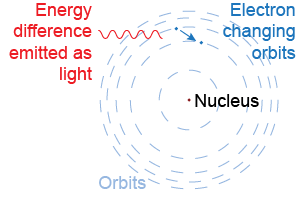 Danish physicist Niels Bohr deduced a brilliant explanation for Balmer’s formula in 1913. Bohr proposed that the electron makes circular orbits around the nucleus. The electron’s energy depends on the radius of the orbit. An electron changing orbits could emit light of specific wavelengths proportional to the energy difference between the orbits. The Bohr model marked the beginning of the quantum theory of the atom.
Danish physicist Niels Bohr deduced a brilliant explanation for Balmer’s formula in 1913. Bohr proposed that the electron makes circular orbits around the nucleus. The electron’s energy depends on the radius of the orbit. An electron changing orbits could emit light of specific wavelengths proportional to the energy difference between the orbits. The Bohr model marked the beginning of the quantum theory of the atom. 
|
| |
|

