|
| Essential questions | | How can you use spectroscopy to identify elements? | |
|
Every atomic element has a different number of protons and electrons, which means that every element has a unique set of energy levels. When an electron makes a transition between energy levels, a photon of light is either emitted or absorbed. Every element’s unique set of energy levels has a corresponding unique set of emission and absorption lines—a signature. In this investigation you will identify unknown elements using their spectroscopic signature. 
|
Part 1: Hand-held visual spectrograph and discharge lamps
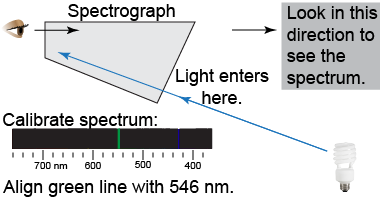 Safety warning: Never point a spectrograph directly at the Sun or any other bright light source. Never touch discharge tubes because they can be very hot.
Safety warning: Never point a spectrograph directly at the Sun or any other bright light source. Never touch discharge tubes because they can be very hot. - Calibrate the hand-held visual spectrograph by pointing it at a fluorescent lamp and adjusting the scale to align the green line with 546 nm.
- Turn on the power for the discharge lamps.
- Point the spectrograph at the fluorescent lamp and discharge lamps.
- What elements can you identify in the overhead fluorescent lamp? Why?
- Based on the spectra you observe, which of the discharge lamps (H, He, Ne, or Ar) is which?

 |
Some lines you might see
Some lines you might see with a hand-held visual spectrograph Wavelength
(nm) | Element | Source |
|---|
| 393 | Calcium | Sunlight reflected off white wall | | 397 | Calcium | Sunlight reflected off white wall | | 405 | Mercury | Fluorescent lamp | | 434 | Hydrogen | Sunlight or discharge lamp | | 436 | Mercury | Fluorescent lamp | | 486 | Hydrogen | Sunlight or discharge lamp | | 517 | Iron | Sunlight reflected off white wall | | 517 | Magnesium | Sunlight reflected off white wall | | 527 | Iron | Sunlight reflected off white wall | | 546 | Mercury | Fluorescent lamp or discharge lamp | | 577 | Mercury | Fluorescent lamp or discharge lamp | | 579 | Mercury | Fluorescent lamp or discharge lamp | | 589 | Sodium | Sunlight or match flame | | 590 | Sodium | Sunlight or match flame | | 656 | Hydrogen | Sunlight or discharge lamp | | 687 | Oxygen | Sunlight reflected off white wall | Source: Project STAR Spectrometer from Learning Technologies, Inc. 
|
Part 2: Identifying mystery elements in spectra
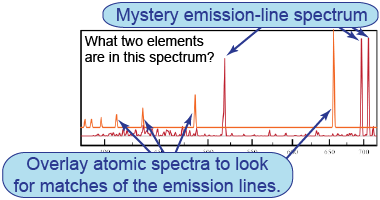
- The first four sheets of emission-line spectra should be printed on clear transparencies. The other pages should be printed on white paper.
- Slide the transparencies over the mystery spectra to identify the element(s) they contain.
- How did you use the known atomic spectra to identify the unknown ones?
- Describe at least three physical properties that can be used to identify elements.

|
Part 3: Identifying elements in stars and the Sun
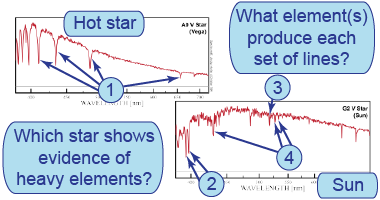
- Use the atomic spectra to identify elements in the absorption lines of various stars.
- How does an absorption-line spectrum differ from an emission-line spectrum?
- Which star(s) show evidence for heavier elements? Why might this happen?

|

