| | Essential questions | | What wavelengths of light are absorbed and/or emitted by a hydrogen atom?
How are these wavelengths related to quantum phenomena? | |
|
In Bohr’s model of the hydrogen atom, the energy levels of the electron are quantized, meaning that they can only take certain, discrete values. For the electron to change energy levels, the atom must either absorb or emit light at specific wavelengths or specific energies. These energies correspond to the difference in the energy levels of the electron. In this interactive simulation, you will shine light onto a hydrogen atom and determine the wavelengths of light that can be absorbed—and then re-emitted—by this hydrogen atom. Only certain photon frequencies (or wavelengths) will be absorbed by the hydrogen atom—the rest will pass through the atom untouched! 
|
Bohr’s model of the hydrogen atom
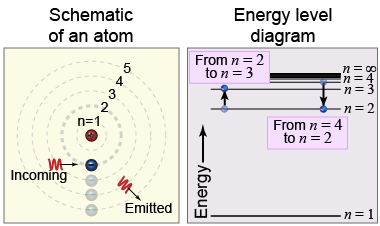 The Bohr model describes a hydrogen atom where only certain energy levels are allowed. To transition between energy levels, the atom must gain or lose the difference in energy between the two levels. If a photon of light with exactly one of these energy differences strikes the hydrogen atom, then the photon may be absorbed—causing a change in the atom’s energy level. Bohr’s model proved to be successful for calculating the absorption and emission line spectra of hydrogen for transitions between n = 2 and higher values of n, called the Balmer series.
The Bohr model describes a hydrogen atom where only certain energy levels are allowed. To transition between energy levels, the atom must gain or lose the difference in energy between the two levels. If a photon of light with exactly one of these energy differences strikes the hydrogen atom, then the photon may be absorbed—causing a change in the atom’s energy level. Bohr’s model proved to be successful for calculating the absorption and emission line spectra of hydrogen for transitions between n = 2 and higher values of n, called the Balmer series. 
|
What wavelengths are absorbed and emitted by the hydrogen atom?
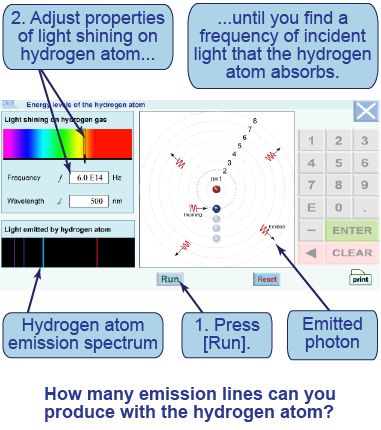
- Launch the interactive simulation and press the [Run] button.
- Adjust the wavelength or frequency of the light. If the light has a wavelength corresponding to an energy level transition, then it will be absorbed—and the hydrogen atom will subsequently emit a photon at the same wavelength.
- Find at least three of the wavelengths that are absorbed by the hydrogen atom.
- What happens when the hydrogen atom absorbs a photon? What happens afterward?
- What are the wavelengths of light that the hydrogen atom could absorb? What energy level transitions do these correspond to?
- Are the wavelengths evenly spread throughout the spectrum? Describe their distribution as a function of wavelength and/or color.
- In this simulation, all the incident photons travel in one direction. What direction do the emitted photons travel? Explain why this happens.

|
In this investigation you will be shining visible light of a particular frequency (or wavelength) onto a hydrogen atom. Only certain photon frequencies (or wavelengths) will be absorbed by the hydrogen atom. Photons having other frequencies will pass straight through the atom untouched!
| |
| |
|

