|
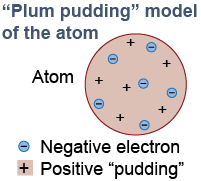 By 1900 it was known that atoms contained tiny negative electrons. Atoms were electrically neutral, and therefore they had to contain an equal amount of positive charge. But it was a complete mystery how the electrons and the positive charge were arranged inside the atom. The prevailing theory, nicknamed the “plum pudding model,” supposed the electrons were sprinkled within a uniform positive substance, like raisins in an English plum pudding.
By 1900 it was known that atoms contained tiny negative electrons. Atoms were electrically neutral, and therefore they had to contain an equal amount of positive charge. But it was a complete mystery how the electrons and the positive charge were arranged inside the atom. The prevailing theory, nicknamed the “plum pudding model,” supposed the electrons were sprinkled within a uniform positive substance, like raisins in an English plum pudding. 
|
In 1909, Ernest Rutherford and Hans Geiger, along with undergraduate student Ernest Marsden, did the crucial experiment in which they shot millions of alpha particles—which were already known to have positive charge—at a thin gold foil. By observing how many alpha particles were scattered at different angles they expected to shed some light on how positive and negative charges were arranged inside the atom. Rutherford expected to find that most of the alpha particles were deflected by very small angles as they “plowed through” the positive “pudding” inside. Instead, they found something wholly unexpected! 
 |
Alpha particles (α particles) are the nuclei of the helium atom and contain two protons and two neutrons. The charge of the α particle is +2 and its mass is well known. Sources of α particles are radioactive elements such as radium and plutonium. Rutherford in his famous scattering experiment in 1909 used radium as the source of α particles. 
|
- Virtually all the alpha particles passed through completely unaffected, as if they had somehow completely missed every atom.
- A very few alpha particles bounced off at large angles and some even bounced backward!

|
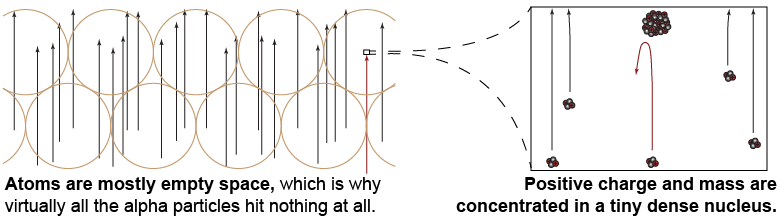
|
From the first observation, the team deduced that most of the space inside an atom must be empty. The second observation could only be explained if nearly all an atom’s mass, and all of the positive charge, were concentrated in a tiny volume, which they called the nucleus. Only a massive positive charge could have bounced a fast alpha particle straight backward. From the ratio of particles that “missed” to ones that “hit” the nucleus Rutherford was able to estimate that the size of the nucleus was 100,000 times smaller than that of the atom! 
|
Inside an atom, neutrons and protons are grouped in a tiny nucleus at the center. The nucleus is extremely small. If the atom were the size of your classroom, the nucleus would be smaller than a grain of sand in the center. Because electrons are so light, 99.97% of an atom’s mass is concentrated in the nucleus. Only 0.03% is in the electrons zipping around the mostly empty space outside the nucleus. 
|

