|
Greek philosophers first proposed the idea of atoms more than 2,500 years ago. It seemed logical that if you kept grinding up matter into smaller bits you should eventually reach a limit as to the smallest possible bit of matter. This they named atomo (άτομο), meaning indivisible. Leucippus’s and Democritus’s intellectual deduction of the atom’s existence was far beyond their ability to prove by experiment. For the next 2,300 years few believed in atoms and the idea remained a minor curiosity. Today the fact that matter is made of atoms is a central theme in our understanding of the universe. We also know that atoms are not indivisible but are themselves composed of even smaller particles—protons, neutrons, and electrons. 
|
The discovery of atomic structure
|
 The modern theory of the atom dates to John Dalton’s 1805 hypothesis that each element is a different kind of atom and that atoms combine together to form compounds. Dalton suggested that atoms of different elements have different masses and sizes. By 1867, the Russian chemist Dmitri Mendeleev had created the first version of the periodic table, which organized the known data about elemental masses and grouped elements by similar properties. Mendeleev’s work was a clue that atoms have an internal structure that is responsible for the different observable properties of the elements.
The modern theory of the atom dates to John Dalton’s 1805 hypothesis that each element is a different kind of atom and that atoms combine together to form compounds. Dalton suggested that atoms of different elements have different masses and sizes. By 1867, the Russian chemist Dmitri Mendeleev had created the first version of the periodic table, which organized the known data about elemental masses and grouped elements by similar properties. Mendeleev’s work was a clue that atoms have an internal structure that is responsible for the different observable properties of the elements. 
|
 The first confirmation of structure in atoms came in 1897 when British physicist J. J. Thomson demonstrated that cathode rays were made of negatively charged particles emitted from matter and were much smaller than atoms. He hypothesized that electrons came from inside the atom. By 1909, Ernest Rutherford of England and New Zealand showed experimentally that nearly all of the mass of the atom is concentrated in a small, dense, central nucleus containing positively charged particles, which he called protons.
The first confirmation of structure in atoms came in 1897 when British physicist J. J. Thomson demonstrated that cathode rays were made of negatively charged particles emitted from matter and were much smaller than atoms. He hypothesized that electrons came from inside the atom. By 1909, Ernest Rutherford of England and New Zealand showed experimentally that nearly all of the mass of the atom is concentrated in a small, dense, central nucleus containing positively charged particles, which he called protons. 
|
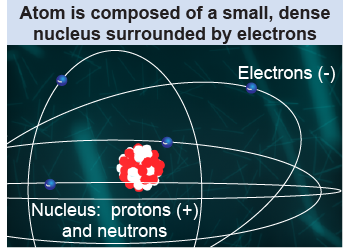 Today, we have a fairly good understanding of the structure of atoms. Atoms contain three particles called electrons, protons, and neutrons. The electron is the lightest of the three and is a small, fast-moving particle with negative electric charge. The proton has positive electric charge and 1,836 times more mass than the electron. The neutron has slightly more mass than the proton but has zero electric charge. The heavy protons and neutrons are located in the nucleus of the atom, while the light electrons can be thought of as whizzing around the outer part of the atom. The nucleus of the atom is so small that, if the nucleus were the size of a grain of rice, the whole atom would be the size of two football fields. That is a small nucleus, indeed!
Today, we have a fairly good understanding of the structure of atoms. Atoms contain three particles called electrons, protons, and neutrons. The electron is the lightest of the three and is a small, fast-moving particle with negative electric charge. The proton has positive electric charge and 1,836 times more mass than the electron. The neutron has slightly more mass than the proton but has zero electric charge. The heavy protons and neutrons are located in the nucleus of the atom, while the light electrons can be thought of as whizzing around the outer part of the atom. The nucleus of the atom is so small that, if the nucleus were the size of a grain of rice, the whole atom would be the size of two football fields. That is a small nucleus, indeed! 
 |
Electrons, protons, and neutrons are usually called indivisible constituents of matter. Since the middle of the 20th century, however, we now know that protons and neutrons consist of even smaller particles called quarks. These quarks are short-lived, in that they cannot survive for very long all by themselves. When three quarks come together a proton or a neutron is formed—both of which are stable and can last for a long, long time. Quarks were discovered in 1968 by breaking up protons using the high-energy particle accelerator at the Stanford Linear Accelerator Center. 
|
Which of the following lists the particles from least massive to most massive? - protons, electrons, atoms, neutrons
- electrons, neutrons, protons, atoms
- electrons, protons, neutrons, atoms
- neutrons, atoms, electrons, protons
 |
The correct answer is c. An atom is made up of electrons, protons, and neutrons. A neutron is slightly more massive than a proton and an electron is the lightest of the three. 
|
| |
|

