|
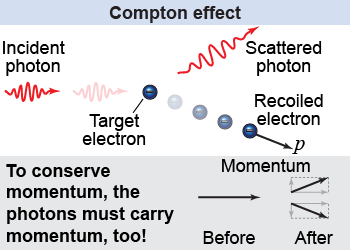 Einstein’s explanation for the photoelectric effect required light to act as a particle, not a wave. Is there any other evidence for the particle nature of light? In 1916, Einstein predicted that photons have momentum. Arthur Compton tested the prediction by scattering x-rays off of electrons. The scattered x-rays decreased in energy, while the recoiling electrons increased in energy and momentum. To conserve total momentum in the collision, the photons must have momentum—a property of particles! The Compton effect further demonstrated that light can act as a particle.
Einstein’s explanation for the photoelectric effect required light to act as a particle, not a wave. Is there any other evidence for the particle nature of light? In 1916, Einstein predicted that photons have momentum. Arthur Compton tested the prediction by scattering x-rays off of electrons. The scattered x-rays decreased in energy, while the recoiling electrons increased in energy and momentum. To conserve total momentum in the collision, the photons must have momentum—a property of particles! The Compton effect further demonstrated that light can act as a particle. 
|
As you have learned in this section, light can exhibit properties of waves and particles. This is called the dual nature of light, and it lies at the heart of modern physics. The traditional physics or classical view of light is that it is a wave, but the atomic view of physics is that light is a particle. 
|
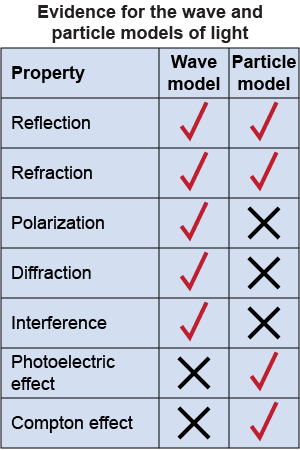
Both the wave and particle models for light can explain that light reflects and refracts when it strikes a boundary between two media. But only the wave model can explain the phenomena of polarization, diffraction, and interference exhibited by light. Young’s double slit experiment in particular provides strong evidence for the wave nature of light. 
| 
|
The wave model fails, however, to explain either the photoelectric effect or the Compton effect. To explain the photoelectric effect, Einstein had to assume that light was made up of particles called photons and that their energies were equal to hf. The explanation for the Compton effect further required photons of light to carry momentum, which is a particle phenomenon. 
|
The new physics of these two experiments demonstrated that the energy of a photon is indivisible; i.e., it cannot be split up into pieces. Photon energies of a given frequency are quantized in that they can only take on a discrete energy known as a quantum. If you shine light of a particular frequency on an object, then your beam of light cannot contain just any amount of energy: It can only contain energy that has integer multiples of the quantum energy. This was the first indication of the new physics of the quantum world. Quantum physics was born! 
|
| |
|

