|
The behavior of electrons in atoms is complex and strange! Why should there be energy levels? Why should the first level hold two electrons, the second hold eight electrons, and so on? What creates the structure reflected in the periodic table and consequentially makes the infinite variety of matter possible, including life? Presenting the mathematics of quantum theory is more than we can do in this book, but we want to provide a conceptual framework for this incredibly successful theory. 
|
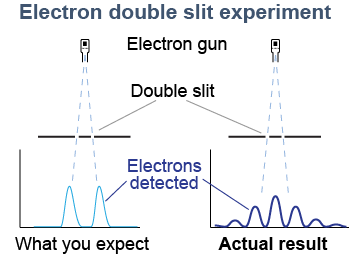 Consider a barrier with two small slits. Electrons are emitted and some pass through the slits and fall on a screen where they are detected. If electrons were classical particles they would be detected in two places, directly in line with each slit. In fact, when the experiment is done with very small slits, something very different happens. We do not see two maxima in front of the slits. Instead, we see an interference pattern that is characteristic of two waves.
Consider a barrier with two small slits. Electrons are emitted and some pass through the slits and fall on a screen where they are detected. If electrons were classical particles they would be detected in two places, directly in line with each slit. In fact, when the experiment is done with very small slits, something very different happens. We do not see two maxima in front of the slits. Instead, we see an interference pattern that is characteristic of two waves. 
|
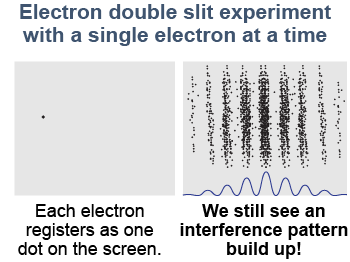 The experiment gets even more interesting if we let only one electron at a time pass through the slits. A single electron is detected at one place on the screen each time the electron gun fires. The weird thing occurs when we record where each electron strikes the screen for 10,000 electrons one at a time. We accumulate the same interference pattern one electron at a time as we did when there were many electrons at once!
The experiment gets even more interesting if we let only one electron at a time pass through the slits. A single electron is detected at one place on the screen each time the electron gun fires. The weird thing occurs when we record where each electron strikes the screen for 10,000 electrons one at a time. We accumulate the same interference pattern one electron at a time as we did when there were many electrons at once! 
|
The one-at-a-time result implies that a single electron somehow passes through both slits at the same time to interfere with itself! Our classical concept of an electron as a particle with a definite position is not adequate to explain the double slit experiment. If the electron is a wave, however, then we can explain the two-slit diffraction pattern by constructive and destructive interference. 
|
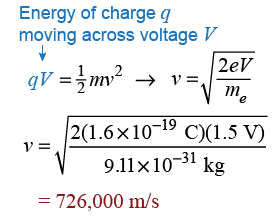 Early experimentalists did not see the electron wave because the slits have to be on the order of the electron wavelength in size. Electrons are so light that a 1.5 V battery will accelerate them to a velocity of 726,000 m/s! At this velocity, the electron wavelength is 1 nanometer (10−9 m). Quantum effects generally become evident when a system is of the order of the electron wavelength.
Early experimentalists did not see the electron wave because the slits have to be on the order of the electron wavelength in size. Electrons are so light that a 1.5 V battery will accelerate them to a velocity of 726,000 m/s! At this velocity, the electron wavelength is 1 nanometer (10−9 m). Quantum effects generally become evident when a system is of the order of the electron wavelength. 
|

