|
How are electrons organized in an atom with many electrons? The fact that each element has a discrete spectra is evidence that all atoms have energy levels. The critical clue to understanding multielectron atoms is found in their chemical behavior. Atoms combine chemically with other atoms by sharing electrons. For example, water (H2O) contains two atoms of hydrogen and one atom of oxygen chemically combined into a molecule. 
|
Dmitri Mendeleev deduced a repeating or periodic pattern in how elements combine. Elements with 3 electrons (Li), 9 electrons (Na), 19 electrons (K), and 37 electrons (Rb) combine with oxygen in 2:1 ratios to make Li2O, Na2O, K2O, and Rb2O. Elements with 2, 10, 18, and 36 electrons are all noble gases (He, Ne, Ar, and Xe) that make no chemical bonds with other elements. Elements with 4 electrons (Be), 12 electrons (Mg), and 20 electrons (Ca) combine with oxygen in a 1:1 ratio to make BeO, MgO, and CaO. This evidence points to some internal structure in the atom that repeats at 2, 10, 18, and 36 electrons.

|
|
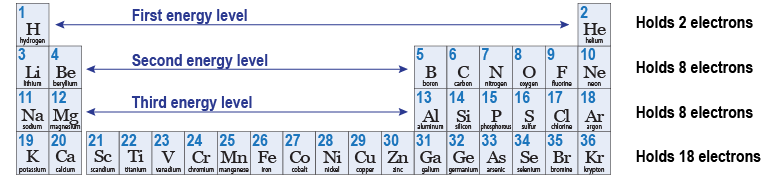
Each energy level holds a fixed number of electrons. The lowest energy level can hold two electrons. A third electron must occupy an open state in the second energy level. The second energy level can hold eight electrons. That means an element with 10 electrons completely fills all the quantum states in the first and second energy levels. The 11th electron has to go into the third energy level. The periodic table is actually a diagram showing how many electrons fit in each energy level. 
|
Neils Bohr proposed that electrons in an atom must occupy stable quantum states. In physics, a “quantum state” refers to a particular allowed set of values for momentum and energy within a system. Think of a small theater with multiple levels of seating. The lowest level has only two seats—corresponding to two allowed quantum states. The second level has eight seats—corresponding to eight allowed quantum states. The third level has eight more seats, and so on. 
|
The Pauli exclusion principle, deduced by Austrian physicist Wolfgang Pauli in 1925, states that no two electrons can be in the same quantum state in the same atom. Once a quantum state is occupied by an electron, all other electrons are “excluded” from that state. The Pauli exclusion principle, combined with Bohr’s quantum states provides the theoretical explanation for the elements. The periodic properties repeat when all the quantum states are filled at one energy level and the next electron starts the next higher energy level. 
|
| |
|

