|
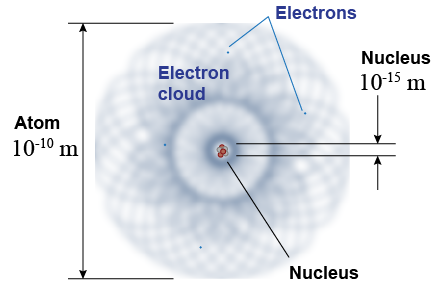
| All atoms have an internal structure of electrons loosely spread around a vast, mostly empty volume with a tiny dense nucleus at the center. The volume of the atom is defined by the electron cloud, which is roughly 10−10 m in diameter. The nucleus is much smaller, of the order of 10−15 m in diameter. Atoms of different elements vary somewhat in size but by less than you might think. More than 99.9% of the mass is in the nucleus. A uranium atom has 240 times the mass of a hydrogen atom and yet its size is only about three times larger. |

|
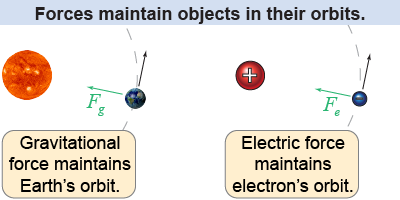 The negatively charged electrons in the cloud are attracted to the positive charge in the nucleus. The force of attraction (from Coulomb’s law) between the protons and electrons binds the electrons to the nucleus. The electrons do not “fall into” the nucleus because they have kinetic energy and momentum. A useful analogy is Earth orbiting the Sun. The Sun’s gravitational force pulls Earth inward, but the planet’s kinetic energy and momentum keeps the Earth on an orbit around the Sun. A similar balance between attraction and momentum keeps electrons out of the nucleus and determines the size of an atom.
The negatively charged electrons in the cloud are attracted to the positive charge in the nucleus. The force of attraction (from Coulomb’s law) between the protons and electrons binds the electrons to the nucleus. The electrons do not “fall into” the nucleus because they have kinetic energy and momentum. A useful analogy is Earth orbiting the Sun. The Sun’s gravitational force pulls Earth inward, but the planet’s kinetic energy and momentum keeps the Earth on an orbit around the Sun. A similar balance between attraction and momentum keeps electrons out of the nucleus and determines the size of an atom. 
|
Gravity may be the most obvious force in everyday life, but it is the electric force that holds matter together beneath the surface. Electric forces also bond atoms to other atoms to create compounds, such as salt. Salt is a compound formed by sodium atoms and chlorine atoms bonded together. While electrostatic forces are not as “forcelike” compared to gravity on the macroscopic scale, these forces are what determine all the underlying properties of matter. 
|
Gravity is intrinsically the weakest of the four known forces of nature. It takes a planet-size large mass to create enough gravity to make a significant force. Electrons, protons, and neutrons have far too little mass for gravity to be significant. The ratio of the gravitational force between an electron and a proton compared to the force of electrostatic attraction is 10−40. This is a very small number. Gravity is so completely insignificant on the scale of atoms that there are no known atomic effects caused by gravity. As a result, we do not have a workable theory for gravity on small scales and this is a major unsolved mystery in physics. 
|
| |
|

