|
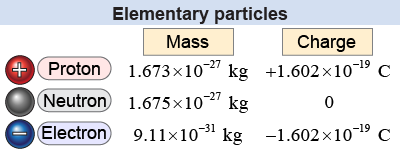 The building blocks of atoms are protons, electrons, and neutrons. The electron is the smallest and least massive, being 1/1,836 the mass of the proton. The proton and neutron have nearly the same mass.
The building blocks of atoms are protons, electrons, and neutrons. The electron is the smallest and least massive, being 1/1,836 the mass of the proton. The proton and neutron have nearly the same mass. 
|
The electron and proton have exactly equal and opposite electric charges. The charge of the electron is −1.602×10−19 coulomb (C) and the charge on the proton is +1.602×10−19 C. The value e = 1.602×10−19 C is called the elementary charge and is represented by a lower-case e. Electric charge only occurs in multiples of the elementary charge. You can have a charge of −e, 0, e, 5e, or 1020e, but nature does not allow charges of 1.5e, −3.3e, or any noninteger multiple of the elementary charge. The neutron has zero electric charge but is important for another force, as we will see on page 757. 
 |
Are the electron, proton, and neutron the only elementary particles in the universe? Hardly! There are numerous other elementary particles, many of which are not stable, meaning that they do not last long before they decay into something else. So it takes diligent research to uncover these other particles before they disappear!
One class consists of elementary particles called leptons. You already know about the electron, but add to that the τ (tau) and μ (muon) particles. Each of those three leptons has a neutrino associated with it: the electron neutrino, the tau neutrino, and the muon neutrino. That’s six particles.
Another class comprises six quarks: up, down, charm, strange, top, and bottom. The proton and neutron are called baryons, but baryons are not elementary particles because the proton and neutron are each made up of a combination of three of these quarks. There are also mesons, such as the π (pion) and κ (kaon), that are made up of one quark and one antiquark.
In addition, every particle has an antiparticle that has the same mass (and spin) but opposite sign for electric charge (if it has a nonzero charge) and quantum numbers. So you can take that list and double it!
But don’t stop there: Bosons make up yet one more class of elementary particles. You know about one boson called light—which is also called a photon—but there is also a W boson, a Z boson, and a gluon. And there are two other bosons: The Higgs boson may have been observed recently, while the graviton has been proposed but not yet observed.
Elementary particles form nothing short of a zoo. We will learn more about them later in the book. 
|
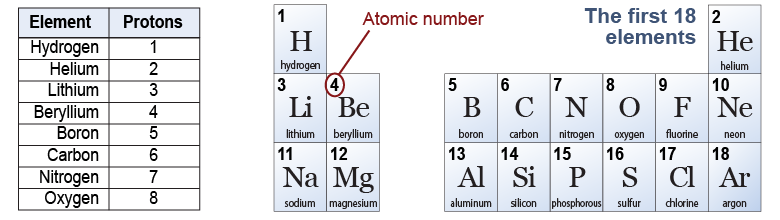
All atoms of the same element have the same number of protons. Atoms of different elements contain different numbers of protons and electrons. For example, every atom of hydrogen has one proton and one electron. Every atom of helium has two protons and two electrons. The number of protons is called the atomic number. The atomic number of carbon is 6, telling you that every atom of carbon has six protons. The lightest element is hydrogen with 1 proton per atom and the heaviest naturally occurring element is uranium with 92 protons per atom.

|
|
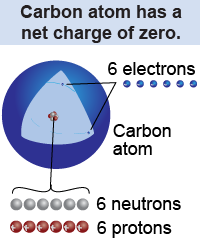 Neutral atoms contain an equal number of protons and electrons. For example, a carbon atom has six protons and six electrons. The elementary charge on the proton and electron are exactly the same, but opposite in sign, and so they cancel each other out and the total charge of an atom is exactly zero. Atoms are electrically neutral, which means that their net charge is zero. The reason we do not ordinarily feel electrical forces in the macroscopic world is not because matter does not contain charge, but because neutral atoms contain the same amount of positive and negative charges.
Neutral atoms contain an equal number of protons and electrons. For example, a carbon atom has six protons and six electrons. The elementary charge on the proton and electron are exactly the same, but opposite in sign, and so they cancel each other out and the total charge of an atom is exactly zero. Atoms are electrically neutral, which means that their net charge is zero. The reason we do not ordinarily feel electrical forces in the macroscopic world is not because matter does not contain charge, but because neutral atoms contain the same amount of positive and negative charges. 
|
A neutral oxygen-18 atom has eight protons and ten neutrons in its nucleus. How many electrons does it have?
 |
The atom is neutral; therefore, the number of electrons equals the number of protons, so there are eight electrons. 
|
| |
|

