|
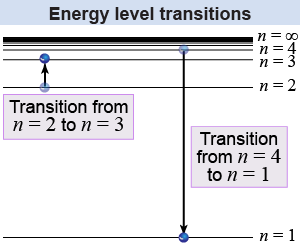
Electrons in an atom can only have an energy that matches one of the atom’s energy levels. Energy levels are the allowed energies within a quantum system, such as an atom. In the hydrogen atom, with one electron, each energy level has a different principal quantum number n, which can be any integer from one to infinity. The principal quantum number is like the floor number in a tall building. The energy level is analogous to the potential energy of the floor, except that the floors are not equally spaced! As the diagram shows, the spacing between energy levels gets smaller as n gets larger. 
| 
|
Atoms change their energy when electrons move up or down between energy levels. For example, an electron at the n = 1 energy level might make a transition to the n = 2 level. Conservation of energy requires that the atom absorb a photon of light with precisely the same energy gained by the electron. Transitions may also skip levels. For example an electron might go from the 23rd energy level to the 5th energy level. The atom conserves energy by emitting a photon with an energy equal to the energy difference between the 23rd level and the 5th level. An electron can even go from n = 1 to n = ∞, which corresponds to the electron being removed altogether from the atom! 
|
 The n = 1 state is the lowest energy level, called the ground state. Atoms in their ground state have the lowest possible energy. The important thing to remember about the ground state is that the electron energy cannot get any lower. From the ground state, the electron can only change to a higher energy level. An excited state is a configuration of the atom in which one or more electrons are in higher energy levels than they could be. Excited states occur when an atom absorbs the energy of a photon.
The n = 1 state is the lowest energy level, called the ground state. Atoms in their ground state have the lowest possible energy. The important thing to remember about the ground state is that the electron energy cannot get any lower. From the ground state, the electron can only change to a higher energy level. An excited state is a configuration of the atom in which one or more electrons are in higher energy levels than they could be. Excited states occur when an atom absorbs the energy of a photon. 
|
The ground state of the hydrogen atom is at an energy of −2.18×10−18 J. A more convenient unit for representing atomic energy levels is the electron volt (eV), which is the amount of energy gained by an electron that moves across one volt. As a result, the electron volt (or eV) is equal to one volt times the charge of the electron, or 1 eV = 1.602×10−19 J. The ground state of the hydrogen atom can be written as (−2.18×10−18 J)/(1.602×10−19 J/eV) = −13.6 eV. Writing the ground state energy as −13.6 eV avoids having to keep track of all those powers of 10! Whenever working with the electron volt, the important point to remember is that it is a unit of energy. 
 |
The electron volt is useful for quantifying the energy of electrons and atoms. It is often applied to the photoelectric effect. Electron volts are useful for representing the work function and how much energy an ejected electron has. 
|
The second energy level (n = 2) of a hydrogen atom is −3.4 eV. If an electron goes from the ground state of a hydrogen atom to n = 2, what is its change in energy? - −17.0 eV
- −3.4 eV
- 3.4 eV
- 10.2 eV
 |
The correct answer is d. The electron gains energy as it rises in energy levels. The amount of energy it gains is equal to the difference between the two energy levels. 
|

