|
The invention of the refrigerator is the reason you can buy fresh fruits and vegetables in February in New England, at a time when no crops are growing outside. Food in a refrigerator cools or freezes because a refrigerator extracts heat from the cold food and rejects the heat into the warmer room. In the refrigerator, heat flows from cold to hot, in the direction opposite to that prescribed by the second law of thermodynamics! This is done through a reversed thermodynamic cycle. 
|
A refrigerator does not violate the laws of thermodynamics because it takes energy input to cause heat to flow from cold to hot. If you unplug your refrigerator, then the situation reverts to normal and heat flows from the warmer room to the colder refrigerator interior. In the reversed thermodynamic cycle work is done on the working fluid instead of by the fluid. 
|
|
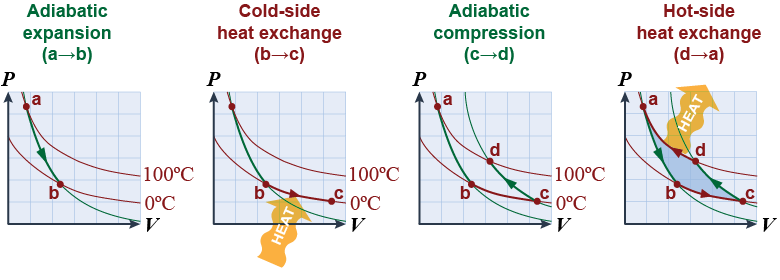
When a fluid is compressed, its temperature increases even if no heat is exchanged. When a fluid is allowed to freely expand, its temperature decreases even if no heat is exchanged. This change in temperature is what allows refrigeration to work. - Consider a reversed gas cycle that starts at 100ºC at point a. The gas is allowed to expand and cool during process a → b.
- During step b → c the 0ºC cold gas absorbs heat because it is colder than the inside of the refrigerator.
- During step c → d the gas is adiabatically compressed and its temperature is raised back to 100ºC.
- During step d → a the gas is now warmer than the room, so it rejects the heat it absorbed during step b → c into the room.
- The cycle is reversed, so the shaded area represents work done on the gas to move the heat from a colder temperature to a hotter temperature.

|
In this example we used a gas. Modern refrigerators, however, operate using a two-phase cycle. The working fluid is liquid during the high-pressure, hot part of the cycle during which heat is rejected to the ambient environment. The expansion that cools the fluid finishes at a low pressure so that the fluid becomes a gas. The cold gas may be −20ºC or lower, which is colder than inside the freezer and therefore able to absorb heat from chilled food. 
|
| |
|

