|
The heat engine is a machine that uses heat to do mechanical work. The operation of a heat engine uses some kind of gas as a working fluid; when that working fluid is heated, its pressure increases and drives the piston in the engine. The thermodynamic processes in a heat engine can be plotted on a pressure–volume or PV diagram. The area enclosed by a thermodynamic process on a PV diagram is the work done. An isothermal process is one in which the temperature is kept constant, whereas an adiabatic process is a typically fast process that has no net exchange of heat. Both processes appear as curves in a PV diagram. The efficiency of all heat engines is less than 100%. The Carnot cycle describes an idealized heat engine that has maximum efficiency, although this efficiency is still less than 100% because the heat is always rejected to a medium at a temperature that is above absolute zero. Refrigerators seem to defy the second law of thermodynamics by taking heat from a cold location and rejecting it into the warmer surroundings. But refrigerators use input energy to do work on the gas or fluid inside them. 
|
|
heat engine, PV diagram, isothermal process, adiabatic process, Carnot cycle, Carnot efficiency, refrigerator
|
|
| | |
|
|
Review problems and questions |
|
- In the first section of this chapter the first law of thermodynamics was described as energy conservation with the equation ΔE = Q + ΔW. But in this section, the operation of a heat engine was described by using an equation with the opposite sign for the last term: ΔE = Q − PΔV. Which is correct? Should it be a positive or a negative sign?


|
Both are correct. In the equations, work done, ΔW, is positive when the work is done on a system but is negative when the work is done by a system. In the heat engine, we were discussing the work done by the heat engine, hence explaining the negative sign! 
|
- In the illustration below, four PV graphs are plotted (I–IV).
- Which plot(s) corresponds to a Carnot engine cycle? Which corresponds to a refrigerator?
- In which plot(s) is the work done by the gas? On the gas?

 |
- Carnot engine: IV. Refrigerator: III (and I, albeit inefficiently!).
- By the gas: II and IV. On the gas: I and III.

|
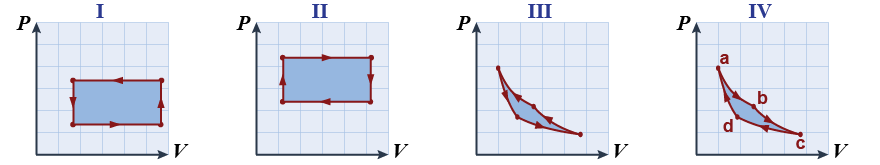
- Refer to the four curved segments (a–b, b–c, c–d, and d–a) in the right-hand illustration (IV) above to answer the following questions.
- Which curved segment(s) correspond to adiabatic processes? Adiabatic compression?
- Which curved segment(s) correspond to isothermal processes? One where heat is rejected?
- Which curved segment(s) are reversible processes? Explain.
- Is the machine or technological process represented by a–b–c–d overall a reversible process?


|
- Adiabatic: b–c and d–a. Adiabatic compression: d–a.
- Isothermal: a–b and c–d. Heat rejected: c–d.
- The adiabatic curves are reversible because no heat flows; therefore, the change in entropy is Q/T = 0. When the change in entropy is positive the process is not reversible because this would violate the second law.
- No, because of the heat flow in the isothermal portions of the process.

|
- A student is analyzing the properties of car’s engine. She knows how much heat flows in the process and the difference in temperature between the hot and cold parts of the Carnot cycle.
- How can she determine the change in entropy with an equation?
- How can she determine the change in entropy graphically?
- She calculates that ΔS is negative. Is this reasonable?

|
- The change in entropy is ΔS = Q/ΔT.
- Draw a TS graph. The total heat is the area inside the cycle on the graph.
- No, because this violates the second law.

|
Take a Quiz |

