|
Entropy gives us a new insight into the meaning of absolute zero. There is precisely one way to organize all the atoms to have minimum energy: A substance at absolute zero is perfectly ordered. The third law of thermodynamics says that absolute zero is the minimum possible temperature; this is the temperature at which the entropy of a system is zero. That means that the system has no ability to transfer heat to any other system by any means. Technically, quantum physics tells us that the real energy can never be exactly zero, but for all practical purposes a system at absolute zero has effectively zero thermal energy. Absolute zero is 0 K, or −273ºC (−459ºF). This is the lower limit of temperature, and no temperature can be lower than absolute zero. 
|

|
Entropy increases extremely rapidly as temperature increases above absolute zero. Entropy also increases with volume. This occurs because each particle can move in three dimensions (x, y, z), each with three possible momentum components (px, py, pz). Each possible variation of position and momentum represents a different “box” that can be occupied by a particle. The more thermal energy there is per particle, the more “boxes” or microstates each particle can potentially occupy. The increase in the number of microstates is what fundamentally creates the increase in entropy. 
|
|
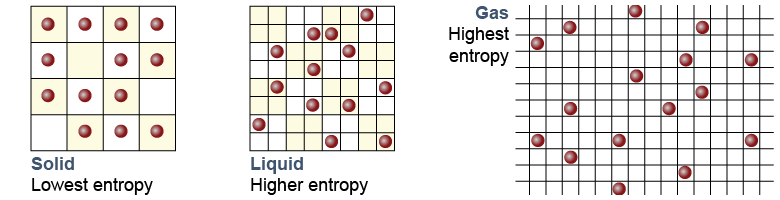
At equal pressure a substance has lower entropy as a solid, higher entropy as a liquid, and even higher entropy as a gas. The entropy difference occurs because molecules in a solid have less energy and therefore fewer degrees of freedom to move around. Although the average spacing of molecules in a liquid is similar to a solid, molecules in a liquid have more thermal energy. More thermal energy means more variation of momentum, increased degrees of freedom, and higher entropy. As a gas, molecules have both more space and more variation in momentum. Gas has the highest entropy of the three ordinary phases of matter. Since entropy changes involve heat, changing phase also requires heat—even if the temperature remains constant. For example, it takes 334 J of heat to turn 1 g of ice at 0ºC into 1 g of liquid water at 0ºC. 
|
| |
|

