|
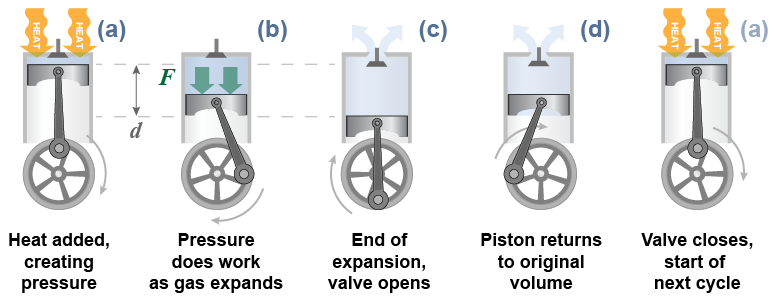
Virtually all heat engines convert heat into mechanical work through the expansion of a gas, referred to as the working fluid. The engine in most cars uses a piston moving in a cylinder. The trapped volume of gas inside the cylinder is the working fluid. - When heat is added, the gas temperature and pressure increase.
- The increase in pressure pushes the piston and does work on the surroundings. As the gas expands, it also cools.
- When the piston reaches the end of its travel, at maximum volume the pressure is released by opening a valve.
- The piston returns to its starting position to begin a new cycle in step a.
The piston is connected to a rotating flywheel that moves the piston back to its starting point.

|
|
The work done by the engine is the force exerted by the gas pressure multiplied by the distance the piston moves. Assume that the piston moves a small distance Δd over which the pressure is approximately constant. The work done is therefore Force is pressure × area. The area × distance is the change in volume ΔV. Therefore another way to write the work done by an expanding gas is as the pressure multiplied by the change in volume: 
|
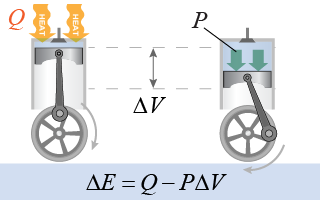 According to the first law of thermodynamics, the change in energy, ΔE, is equal to the total heat Q added to the system minus the work W done by the system. (Alternatively, you could add the work done on the system.) You might think that it could be theoretically possible to invent an engine that would take in 100 J of heat and do 100 J of output work. Over the next few pages we will see that this is impossible!
According to the first law of thermodynamics, the change in energy, ΔE, is equal to the total heat Q added to the system minus the work W done by the system. (Alternatively, you could add the work done on the system.) You might think that it could be theoretically possible to invent an engine that would take in 100 J of heat and do 100 J of output work. Over the next few pages we will see that this is impossible! 
|
| |
|

