|
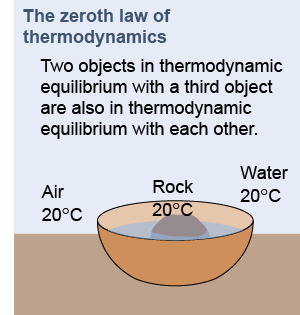 Two objects in thermodynamic equilibrium with a third object are also in thermal equilibrium with each other. For example, suppose the air temperature is 20ºC surrounding a stone placed next to a bowl of water. The stone and water eventually come to thermal equilibrium with the air at 20ºC. Suppose you now drop the 20ºC stone into the 20ºC water. The zeroth law of thermodynamics says that no heat will flow between them because both are at the same temperature. That is the real meaning of the zeroth law—that heat cannot naturally flow between any two objects at the same temperature.
Two objects in thermodynamic equilibrium with a third object are also in thermal equilibrium with each other. For example, suppose the air temperature is 20ºC surrounding a stone placed next to a bowl of water. The stone and water eventually come to thermal equilibrium with the air at 20ºC. Suppose you now drop the 20ºC stone into the 20ºC water. The zeroth law of thermodynamics says that no heat will flow between them because both are at the same temperature. That is the real meaning of the zeroth law—that heat cannot naturally flow between any two objects at the same temperature. 
|
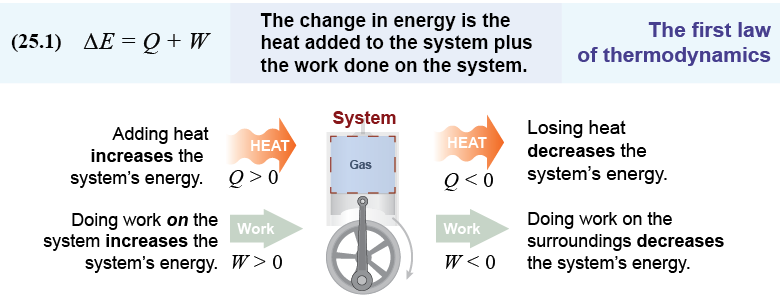
The first law of thermodynamics is the law of energy conservation applied to processes that involve heat energy and at least one other form of energy, such as work. The work done by a system can be no more than the heat lost by the system. If a system cools and loses 100 J of thermal energy by any process, the most you can ever expect is 100 J of mechanical work. Another way to interpret the first law of thermodynamics is to think of heat as simply another form of energy subject to the same conservation law as potential energy, kinetic energy, and work. 
|
|
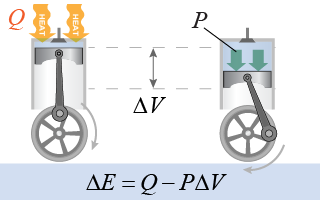 The engine in a car and the steam turbine in a power station involve converting heat into mechanical work through the expansion of a gas. To apply thermodynamics to these systems it is useful to express the work done, W, in terms of pressure P and volume V. Recall that work is the product of force and distance, which is equivalent to the product of pressure and volume. If a gas experiences a volume change ΔV, then the work done by the system is the product of the pressure and the change in volume: W = −PΔV. The negative sign means that this is work done by the system.
The engine in a car and the steam turbine in a power station involve converting heat into mechanical work through the expansion of a gas. To apply thermodynamics to these systems it is useful to express the work done, W, in terms of pressure P and volume V. Recall that work is the product of force and distance, which is equivalent to the product of pressure and volume. If a gas experiences a volume change ΔV, then the work done by the system is the product of the pressure and the change in volume: W = −PΔV. The negative sign means that this is work done by the system. 
|
| |
|

