|
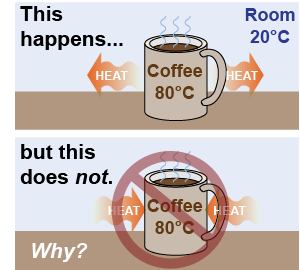 We observe that heat naturally flows from higher temperature to lower temperature and never the other way around. If a cup of coffee at 80ºC is left in a 20ºC room, heat always flows from the coffee to the room. We do not observe the opposite behavior. We never see heat flow from the cooler air into the warmer coffee. Why? Newton’s laws of motion allow things to go forward or backward equally. Why does heat only flow in one direction, from higher temperature to lower temperature?
We observe that heat naturally flows from higher temperature to lower temperature and never the other way around. If a cup of coffee at 80ºC is left in a 20ºC room, heat always flows from the coffee to the room. We do not observe the opposite behavior. We never see heat flow from the cooler air into the warmer coffee. Why? Newton’s laws of motion allow things to go forward or backward equally. Why does heat only flow in one direction, from higher temperature to lower temperature? 
|

|
The ability to go equally forward or backward is called reversibility. A reversible process may proceed identically in the other direction when the inputs are reversed. If cooling were reversible, it would be possible for a 20ºC coffee to warm back up to 80ºC by exchanging heat with room air. The room air certainly has sufficient thermal energy, so energy conservation is not the reason. We observe, however, that any process in which heat flows is irreversible! Why? Why is heat flow irreversible? 
|
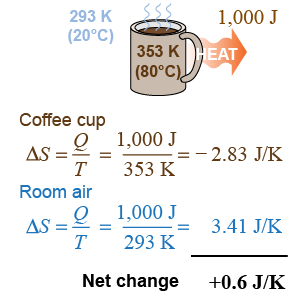 Suppose the cup loses Q = 1,000 J of heat at a temperature T = 80ºC. Divide the heat by the temperature and you get a new property, S = Q/T, called entropy and having units of energy per degree. The coffee cup loses 2.8 J/K of entropy while the room air gains 3.4 J/K for a net change of +0.6 J/K. The second law of thermodynamics is really about entropy; entropy determines whether a process is reversible. If the cooling were to reverse, then the net change in entropy would be −0.6 J/K, but processes that decrease total entropy do not occur spontaneously!
Suppose the cup loses Q = 1,000 J of heat at a temperature T = 80ºC. Divide the heat by the temperature and you get a new property, S = Q/T, called entropy and having units of energy per degree. The coffee cup loses 2.8 J/K of entropy while the room air gains 3.4 J/K for a net change of +0.6 J/K. The second law of thermodynamics is really about entropy; entropy determines whether a process is reversible. If the cooling were to reverse, then the net change in entropy would be −0.6 J/K, but processes that decrease total entropy do not occur spontaneously! 
|

|
- A process is reversible only if entropy remains constant, i.e., ΔS = 0.
- The total entropy of a closed system always increases if any energy is exchanged as heat, i.e., ΔS > 0.
- All naturally occurring processes only proceed in the direction that increases total entropy.

|
| |
|

