|
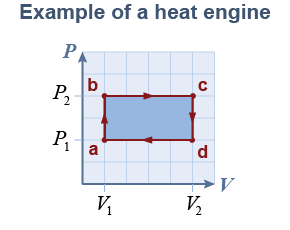 The efficiency of a heat engine is the ratio of work output divided by heat input. The process from a to b occurs at constant volume; the heat added, Qab, therefore depends on the moles of gas, the molar specific heat at constant volume, CV, and the change in temperature. The process from b to c occurs at constant pressure, so the heat added, Qbc, instead depends on the molar specific heat at constant pressure, CP:
The efficiency of a heat engine is the ratio of work output divided by heat input. The process from a to b occurs at constant volume; the heat added, Qab, therefore depends on the moles of gas, the molar specific heat at constant volume, CV, and the change in temperature. The process from b to c occurs at constant pressure, so the heat added, Qbc, instead depends on the molar specific heat at constant pressure, CP: 
|
The equations above and below use the molar specific heat—which you learned about on page 670. Typical values for air are CV = 20.8 J/(mol K) and CP = 29.1 J/(mol K). 
|
Assume an ideal gas for which PV = nRT. The temperatures at points a, b, c, and d are therefore determined by the pressures and volumes: 
|
After substituting for the temperatures we find the heat inputs Qab and Qbc: 
|
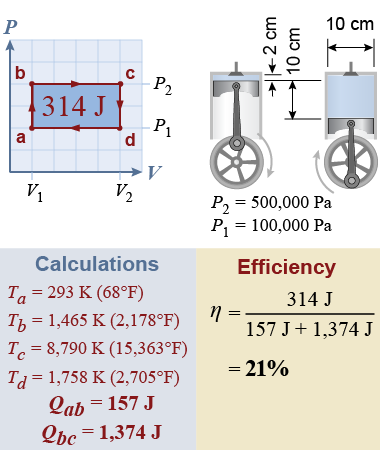 Consider a large car engine, in which each piston has a 10 cm diameter, stroke of 10 cm, and clearance of 2 cm and operates between 1 and 5 atmospheres (100,000 to 500,000 Pa). Apply the above equations to calculate the input heat as The work done in the thermodynamic process is the area inside the PV diagram, or The input heat is almost five times the work output of the engine! Furthermore, to keep the pressure constant from b to c requires that the temperature be Tc = 15,363ºF! A real engine could not sustain this high a temperature.
Consider a large car engine, in which each piston has a 10 cm diameter, stroke of 10 cm, and clearance of 2 cm and operates between 1 and 5 atmospheres (100,000 to 500,000 Pa). Apply the above equations to calculate the input heat as The work done in the thermodynamic process is the area inside the PV diagram, or The input heat is almost five times the work output of the engine! Furthermore, to keep the pressure constant from b to c requires that the temperature be Tc = 15,363ºF! A real engine could not sustain this high a temperature. 
|
This thermodynamic cycle has a terrible efficiency of 21%. Heat not converted to work is rejected to the surroundings during steps c → d and d → a. According to the first law, the heat rejected is the heat added minus the work done, or 1,271 J (79%). Soon we will see how to get better efficiency, but, according to basic thermodynamic principles, no heat engine operating above absolute zero can convert 100% of heat into output work. 
|
| |
|

