|
Heat flows only when there is a temperature difference, Thigh > Tlow. When heat is exchanged between two temperatures, the quantity S = Q/T summed for both sides of the exchange will always increase because the ratio Q/Thigh is always less than the ratio Q/Tlow. The numerator Q is the same and the denominator is different, with Tlow less than Thigh. 
 |
The only exception would be if heat could flow in such a way that ΔT = 0. Just as we approximate low-friction systems by assuming zero friction, it is often useful to approximate some processes in thermodynamics as reversible. Later in this chapter we will assume that heat is reversibly exchanged across a series of infinitesimally small temperature differences to evaluate the ideal efficiency of a perfect machine. Although reversible heat exchanges are convenient and useful in theory, they do not actually occur in real life. 
|
Since the ratio Q/T always results in a net increase, you might reason that we have not proved anything by inventing this new property called entropy. You might be right if that were the whole story! But entropy involves a much deeper concept that lies at the very heart of our modern understanding of physics. While we used the coffee cup argument to introduce the concept, we have not yet explained why entropy must increase and what entropy represents. 
|
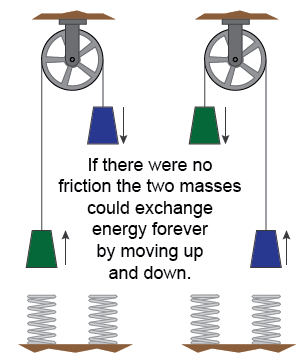 The concept of reversibility is fundamental to the physics of time. Consider two equal masses connected by a string passing over a frictionless pulley. Completely elastic springs on either side give each mass a bounce back upward when it touches down. Now imagine starting the masses moving and recording a video. In a reversible, frictionless world the masses would bounce up and down, exchanging potential and kinetic energy forever. The video would look exactly the same running forward or running in reverse. In a frictionless, reversible world forward or backward in time are equivalent and equally possible.
The concept of reversibility is fundamental to the physics of time. Consider two equal masses connected by a string passing over a frictionless pulley. Completely elastic springs on either side give each mass a bounce back upward when it touches down. Now imagine starting the masses moving and recording a video. In a reversible, frictionless world the masses would bounce up and down, exchanging potential and kinetic energy forever. The video would look exactly the same running forward or running in reverse. In a frictionless, reversible world forward or backward in time are equivalent and equally possible. 
|
Now consider the impact of friction. Once heat flows, ΔS > 0 and some of the energy of motion is irreversibly transformed into heat with each bounce. The second bounce is a little slower than the first. The third is a little slower than the second. On a videotape there is a measurable difference between forward and backward. Irreversibility causes a difference between time moving forward and backward. In the statement that total entropy must always increase, the second law of thermodynamics fixes the direction of time as forward, in the direction of increasing entropy. 
|
In Chapter 23, we talked about temperature and the particle nature of matter. When particles interact on the atomic scale energy and momentum are perfectly conserved and therefore each interaction is reversible. Time does not have a forward preference in the frictionless quantum world. The irreversibility arises when we consider 1023 particles on the macroscopic scale. Although there is no fundamental law against reversibility, the chance of a process involving so many particles randomly reversing itself is so low it will never occur in a thousand times the age of the universe. Entropy is fundamentally related to order and the second law is fundamentally a statistical argument that systems that are disordered do not spontaneously go back into highly ordered states. 
|

