|
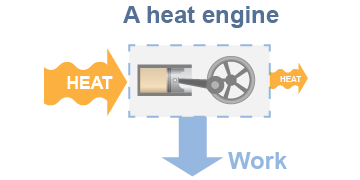 A heat engine is a system that converts thermal energy, or heat, into other forms of energy, usually mechanical work. This includes the gasoline or diesel engine in any modern car and also the enormous turbines that spin the generators in every nuclear-, gas-, or coal-powered electric generating station. In this section you will learn the fundamental physics behind all heat engines, including why a 100% efficient heat engine is impossible.
A heat engine is a system that converts thermal energy, or heat, into other forms of energy, usually mechanical work. This includes the gasoline or diesel engine in any modern car and also the enormous turbines that spin the generators in every nuclear-, gas-, or coal-powered electric generating station. In this section you will learn the fundamental physics behind all heat engines, including why a 100% efficient heat engine is impossible. 
|
Heat engines
|
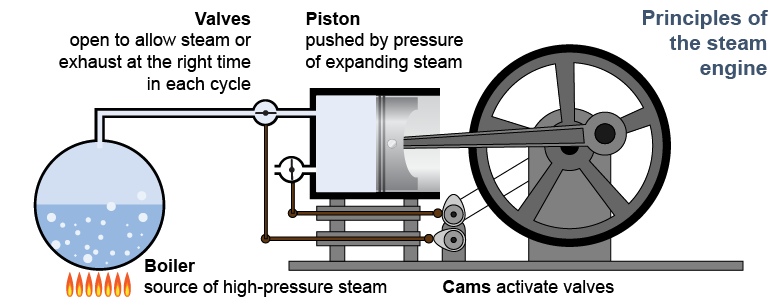
Newcomen’s 1712 steam engine was the first industrial heat engine. Its efficiency, however, was very low and the engine burned a lot of coal. In 1763, Scottish instrument maker James Watt was called upon to repair one of Newcomen’s engines. Instead, Watt devised a much-improved engine. Over the next decade Watt’s improvements to the steam engine were so successful that the unit of power (the watt) is named after him. “Inventor of the steam engine” and “James Watt” are nearly synonymous today. Modern heat engines, including Watt’s design, use pressure rather than vacuum to provide the mechanical force that converts thermal energy into work.

|
|
Conceptually, all heat engines extract energy from the flow of heat between a higher temperature and a lower temperature. It looks simple on a diagram. In practice, however, this is a very complex process involving moving pistons or spinning turbines. Real heat engines convert heat to mechanical energy by manipulating the pressure, volume, temperature, and/or phase of a working fluid. In a car engine the working fluid is mostly air. Heat comes from the ignition of a gasoline/air mixture. Energy is extracted by the work done as the hot (air/gas) working fluid pushes against a piston, expanding its volume. In an electric generating station the working fluid is steam. Heat may come from nuclear reactions, gas, or coal. In all three cases the heat is used to boil water into high-pressure steam, which expands by spinning a turbine, which turns an electric generator. 
|
| |
|

