|
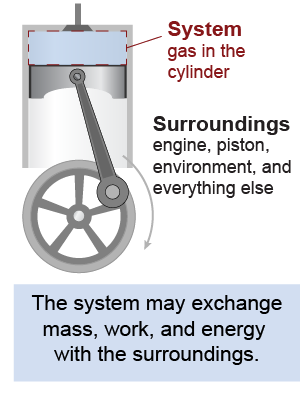 Thermodynamics is usually applied to analyze the behavior of a system as it exchanges matter or energy with the surroundings. The system includes matter we are interested in that may be taking in or giving up energy. The surroundings are everything else. For example, a piston engine traps a volume of gas in a cylinder. The gas is the system. The gas may absorb heat from a fire, or it may exchange work with the surroundings when the piston moves. To simplify the analysis we often assume the surroundings are an infinite reservoir of heat and therefore may exchange work and energy with the system without a change in temperature or pressure. This is valid as long as the surroundings are “large” compared to the system.
Thermodynamics is usually applied to analyze the behavior of a system as it exchanges matter or energy with the surroundings. The system includes matter we are interested in that may be taking in or giving up energy. The surroundings are everything else. For example, a piston engine traps a volume of gas in a cylinder. The gas is the system. The gas may absorb heat from a fire, or it may exchange work with the surroundings when the piston moves. To simplify the analysis we often assume the surroundings are an infinite reservoir of heat and therefore may exchange work and energy with the system without a change in temperature or pressure. This is valid as long as the surroundings are “large” compared to the system. 
|
In thermodynamics, any change in a system occurs through a process. We distinguish “process” from “change” because there are many different ways in which a particular change can occur. For example, you can add heat to a gas and keep the volume fixed. Both temperature and pressure increase in this process. You can also add the same amount of heat while letting the gas freely expand. This is a different process and the results are different. 
|
|
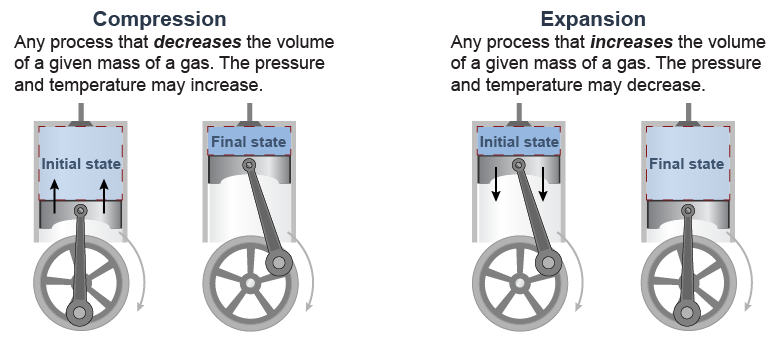
Two common processes involve expansion and compression. In expansion, the volume of a system increases. In the diagram above, expansion occurs when the piston moves down, making the volume of gas in the cylinder larger. Compression is the opposite process. In compression, the volume of the system decreases. In the diagram, compression occurs when the piston moves up, reducing the volume of the trapped gas in the cylinder. The actual quantity of matter may remain the same during expansion or compression! Gases expand and contract to fill their container. 
|
| |
|

