|
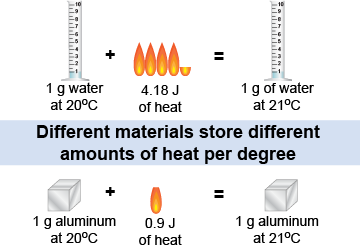 Different materials contain different amounts of thermal energy, even when they are at the same temperature. For example, it takes 4,184 J of energy to raise the temperature of 1 kg of water by 1°C. By comparison, it only takes 900 J to raise the temperature of 1 kg of aluminum by 1°C. The quantity of matter (1 kg) is the same, but the change in thermal energy per degree of temperature is different.
Different materials contain different amounts of thermal energy, even when they are at the same temperature. For example, it takes 4,184 J of energy to raise the temperature of 1 kg of water by 1°C. By comparison, it only takes 900 J to raise the temperature of 1 kg of aluminum by 1°C. The quantity of matter (1 kg) is the same, but the change in thermal energy per degree of temperature is different. 
|
The amount of thermal energy per degree per kilogram is called the specific heat. Water has a specific heat of 4,184 J/(kg °C), which means that water contains 4,184 J of thermal energy per degree per kilogram. Aluminum has a lower specific heat of 900 J/(kg °C) and contains 900 J of thermal energy per degree per kilogram. Thermal energy (as well as heat) depends on temperature, mass, and specific heat. 
|
The change in heat Q for an object depends on its mass m and its change in temperature from T1 and T2, as given by equation (23.6). The specific heat cp is determined by the material and can vary widely. The capital letter Q is traditionally used to represent thermal energy or heat, even though the letter E is used to represent other forms of energy. 
|
| (23.6) | | | Q | = | thermal energy or heat (J) | | m | = | mass (kg) | | cp | = | specific heat (J kg-1 °C-1) | | T1, T2 | = | temperature (K or °C) | | ΔT | = | change in temperature (K or °C) = T2 − T1 |
| Specific heat
|
|
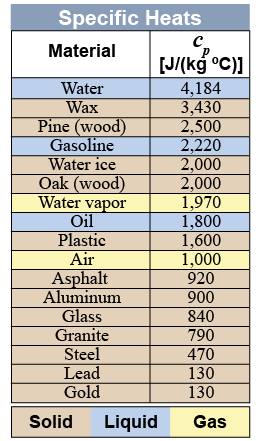
The specific heat of ordinary matter varies from about a hundred to a few thousand joules per kilogram and per degree Celsius. The table on the right gives some examples for common solids, liquids, and gases. Solid metals have the lowest specific heat. Heavier atoms (or denser materials) typically have lower specific heats. 
| 
|
Liquid water has the highest specific heat of common substances. The H2O molecule is fairly light, and intermolecular forces between water molecules are unusually strong because the water molecule is polar. Notice that the specific heat of water is quite different depending on whether the phase is solid, liquid, or gas. Specific heat depends on the strength of interactions between molecules. Ice is less dense than liquid water, so ice molecules are farther apart than liquid water molecules. The extra distance reduces the strength of the intermolecular forces. 
|
Some chemistry textbooks prefer to express the molar specific heat in joules per mole per kelvin, rather than use the specific heat (which is in joules per kilogram per kelvin). Always keep track of your units to make sure you are using the correct quantity! 
|
How much heat does it take to raise the temperature of 3 kg of aluminum by 10°C? - 9,000 J
- 27,000 J
- 480,300 J
- 27,000,000 J
 |
Answer b is correct. 
|

