- Which of the following instruments does not act as a spectrograph?
- a glass prism
- a gas discharge tube
- a transmission diffraction grating
- a hand-held visual spectroscope
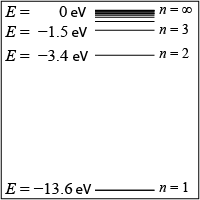
- In the energy level diagram shown, how much energy will the atom emit in the form of a photon if its electron moves from the n = 3 to the n = 1 energy level?
- 1.5 eV
- 1.9 eV
- 12.1 eV
- 13.6 eV
- Which phenomenon requires the quantization of light in order to explain it?
- electromagnetic radiation
- Rutherford scattering
- the photoelectric effect
- alpha decay
- In a hydrogen atom with radius 2.5×10−11 m, what is the electric force of attraction between the proton and the electron? (The Coulomb constant is 9.0×109 N m2/C2 and the elementary charge is 1.602×10−19 C.)
- 3.7×10−7 N
- 2.2×1026 N
- 58 N
- 4.1×10−17 N
- An atom has an energy of −16 eV for its ground state, whereas its first excited state is at −4 eV. Photons with energy of 6 eV bombard the atom while it is in its ground state. What will happen to the atom?
- Nothing will happen.
- The atom’s electron will absorb one photon and transition to the first excited state.
- The atom’s electron will absorb two photons and transition to the first excited state.
- The atom’s electron will absorb one photon and immediately re-emit a photon with the same energy.
| | - Johann Balmer proposed a formula that could be used to predict quantitatively the wavelengths of the emission lines of hydrogen in the visible portion of the electromagnetic spectrum. What physical intuition was behind his formula?
- Electrons act as both a particle and a wave.
- The energy levels of an atom are quantized.
- The speed of light is constant in all reference frames.
- The position and velocity of an electron cannot be perfectly determined simultaneously.
- The electron of a hydrogen atom changes its level from n = 1 to n = 2. What is true of the atom?
- The atom emitted a photon of energy equal to the energy of the n = 2 level.
- The atom absorbed a photon of energy equal to the energy of the n = 1 level.
- The atom emitted a photon of energy equal to the difference between the two energy levels.
- The atom absorbed a photon of energy equal to the difference in energy between the two energy levels.
- An experimenter illuminates an incandescent light bulb and a hydrogen gas discharge tube and passes the light of each through a prism. More colors are observed from the incandescent bulb than from the hydrogen gas. Why?
- The incandescent bulb is hotter.
- The gas in the incandescent bulb is rarefied.
- The hydrogen gas is hotter.
- Only certain atomic transitions can be made by the hydrogen gas.
- What historical experiment demonstrated that the nucleus is massive and occupies a tiny fraction of the volume of the atom?
- Young’s double slit experiment
- Rutherford’s scattering experiment
- Millikan’s oil drop experiment
- Hertz’s photoelectric effect experiment
- The particles that orbit the nucleus are
- protons.
- neutrons.
- electrons.
- compounds.
- The production of light by the laser in a laser pointer is based on the concept of
- stimulated emission.
- stimulated absorption.
- spontaneous emission.
- stimulated absorption.
|

