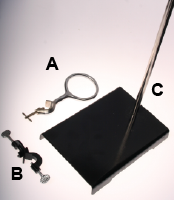
- Identify the three pieces of equipment shown: ring stand, ring clamp, and 90° clamp.
- Why shouldn’t you place a styrofoam cup directly on a hot plate?
- Some of the older Celsius thermometers contain mercury. What precautions should be taken when using them?
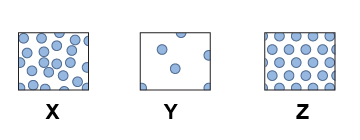
- The three diagrams describe the phases of matter in which order?
| a. | X: solid, | Y: liquid, | Z: gas | | b. | X: gas, | Y: liquid, | Z: solid | | c. | X: liquid, | Y: solid, | Z: gas | | d. | X: liquid, | Y: gas, | Z: solid |
- Which of the following is the best description of the concept of specific heat?
- Specific heat is the number of Celsius degrees of temperature change per kilogram of matter.
- Specific heat is the energy needed to raise one kilogram by one degree Celsius.
- Specific heat is the mass in kilograms of one cubic meter of matter at 0ºC.
- Specific heat is the temperature change in degrees Celsius when one kilogram of matter absorbs one joule of heat.
 A Fahrenheit degree represents how much temperature change compared to a Celsius degree? A Fahrenheit degree represents how much temperature change compared to a Celsius degree? - less
- more
- the same amoung
- one degree
| |  Describe a situation in which two objects have the same temperature but contain different amounts of heat. Describe a situation in which two objects have the same temperature but contain different amounts of heat.
 Object A has twice the specific heat of Object B. A candle flame applies the same amount of heat to both objects. Which of the following is true and why? Object A has twice the specific heat of Object B. A candle flame applies the same amount of heat to both objects. Which of the following is true and why? - Object A is warmer than Object B.
- Object B is warmer than Object A.
- Object A and Object B are at the same temperature.
- It is impossible to say without knowing the masses of the objects.
 The caloric theory explains temperature and heat with the hypothesis that temperature measures the quantity of a substance called caloric that is present in objects proportional to their heat content and temperature. In this theory, warmer objects contain more caloric than colder objects. The theory must account for the following pieces of empirical evidence: (1) Heat flows from hot to cold but not from cold to hot. (2) An object has the same mass when hot as it does when cold. The caloric theory explains temperature and heat with the hypothesis that temperature measures the quantity of a substance called caloric that is present in objects proportional to their heat content and temperature. In this theory, warmer objects contain more caloric than colder objects. The theory must account for the following pieces of empirical evidence: (1) Heat flows from hot to cold but not from cold to hot. (2) An object has the same mass when hot as it does when cold.
- Evaluate the caloric explanation of temperature, using logical reasoning to argue why one of the pieces of evidence is consistent with the caloric theory and one is not.
- Suppose experimental testing could only measure mass to the nearest 0.1 g. How might that affect whether the observations confirm or refute the caloric theory?
- A student places a beaker of hot water on a sensitive balance. The balance shows that the mass decreases as the water cools off. The student argues that this evidence supports the caloric theory because the lost mass represents the flow of caloric out of the cooling water. Analyze and critique the student’s reasoning. (Is there another possible explanation for the reduction in mass?)
- Propose an investigative procedure to evaluate the caloric explanation with a better experimental test in which the alternative explanation in Part c is not an important factor. What is your testable hypothesis?
 If the temperature of a fixed volume of gas increases, and the quantity (mass) of the gas stays the same, what must happen? If the temperature of a fixed volume of gas increases, and the quantity (mass) of the gas stays the same, what must happen? - The pressure must decrease.
- The pressure must increase.
- The gas must condense into a liquid.
- The gas molecules break apart into separate atoms
|

