| | Essential questions | | How do we explain how the same substance can be a solid, liquid, or gas
at different temperatures? | |
|
Temperature measures the kinetic energy that comes from random motion of individual atoms (or molecules). In matter, atoms are attracted to neighboring atoms by intermolecular forces. These attractive forces create potential energy, much like springs. Whether matter is solid, liquid, or gas depends on the balance between the attractive forces between neighboring atoms and the agitation of thermal energy that tends to separate atoms from each other. 
|
Simulating the phases of matter
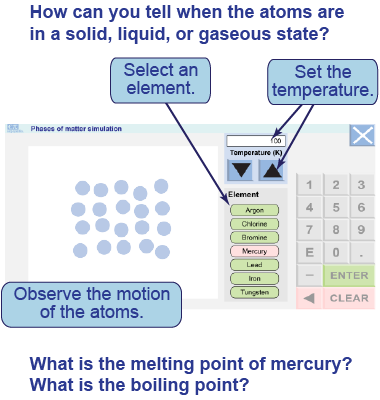 The interactive model simulates a collection of magnified atoms in slow motion. You may choose different elements and set the temperature (in kelvins) using numbers or the arrow buttons.
The interactive model simulates a collection of magnified atoms in slow motion. You may choose different elements and set the temperature (in kelvins) using numbers or the arrow buttons. - Choose mercury as an element.
- Watch the simulation for temperatures of 200 K (−73ºC), 300 K (27ºC), and 1,000 K (727ºC). You may have to watch for a few minutes as the atoms reach equilibrium at the chosen temperature.
- Try the simulation for different elements.
- What different behaviors of the atoms in the model do you associate with the phases of solid, liquid, and gas?
- How can you use the visual behavior of the atoms in the simulation to determine the melting point of an element? The boiling point?
- Determine the approximate melting and boiling points for the elements chlorine, bromine, mercury, iron, lead, and tungsten. Tabulate your results.
- Research the melting and boiling points for each of these elements, as well as for argon. Convert the data to kelvins and compare them to what you observed in the simulation.
- Why is it difficult to determine the melting and boiling point for argon using this simulation?
- By referring to what you learned from this simulation, describe the connection between energy as viewed at the macroscopic scale and the motions of the particles.

|
|
In this interactive simulation, you will simulate the role temperature plays in transitioning among the three phases of matter.
|
Understanding this simulation
This simulation uses 20 atoms in two dimensions. Real matter contains 1020 or more atoms in three dimensions and the atoms move much faster. Like all models, the approximations included in the simulation approximate behavior that is similar to, but not exactly like, the behavior of real atoms in matter. 
|
| |
|

