|
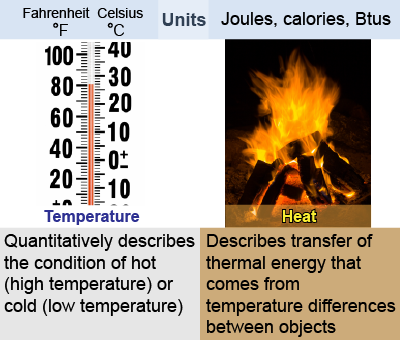 The words heat and temperature are often used interchangeably in conversation, but in physics they are different.
The words heat and temperature are often used interchangeably in conversation, but in physics they are different. - Temperature describes the average kinetic energy in random thermal motion per atom or molecule.
- Heat and thermal energy describe the total amount of energy resulting from temperature in a quantity of matter containing many atoms or molecules.
More specifically, thermal energy is the energy associated with temperature, whereas heat is thermal energy that can be transferred from one object to another owing to their difference in temperature. For example, heat flows from a hotter object to a colder object. 
|
 Heat is a form of energy and is therefore measured in joules. For historical reasons, however, there are many other units for heat.
Heat is a form of energy and is therefore measured in joules. For historical reasons, however, there are many other units for heat. - One calorie is 4.184 J.
- One food Calorie is 1 kilocalorie (kcal) or 4,184 J.
- One British thermal unit (Btu) is 1,055 J.
Nutrition labels list the energy content of foods in kilocalories: Calories with an uppercase “C”! Home heating systems and fuels are rated in Btus. For example, a typical home heating furnaces might deliver 100,000 Btus of heat per hour. 
|
Nutritional labels use Calories as the unit of energy, so how many joules of energy are there in 1 Calorie? Start with 1 Calorie and use the unit conversions between Calories/calories and calories/joules to convert a Calorie into joules: | |

|
All forms of energy can be transformed into heat. A common example is heat generated through friction. Work done against friction is partially transformed into heat. For example, a friction force of 1 N acting over 1 m converts 1 J of work into heat. Moving parts get warm and can even melt from the heat generated through friction. 
|
A liter of gasoline releases 35,000,000 J of chemical energy when completely burned with oxygen. This chemical energy is almost entirely released from the burning reaction in the form of heat. Most of the heat (65%) flows out of the radiator and tailpipe. On average, only about 13% of the heat released from the fuel goes toward physically moving the car. 
|
Which of the following objects has the most thermal energy? - boiling water
- ice
- liquid nitrogen
- The answer cannot be determined without more information.
 |
The answer is d. Thermal energy is the total energy resulting from temperature, so it will depend on the mass of the substance as well as the temperature—as we will learn in more detail on the next page. Although boiling water has the highest temperature, if there is a great mass of ice and only a small mass of boiling water, then the ice could have more thermal energy. 
|

