|
Boyle’s law, Charles’s law, and Gay-Lussac’s law were discovered experimentally. Our modern understanding of matter combines all three equations into the ideal gas law in equation (23.8). An ideal gas is an imaginary form of matter consisting of randomly moving particles that have mass but zero volume. These particles interact with each other only by perfectly elastic collisions that exchange energy and momentum among the particles and any walls confining the gas. Practically speaking, atoms are so small that real gases behave like ideal gases at low pressures (such as at one atmosphere) and at temperatures significantly above the material’s boiling point. 
 |
A gas obeys the ideal gas law when its particles are relatively far apart and do not interact strongly. Highly compressed gases, or gases at temperatures near their boiling point, do not obey the ideal gas law. Some gases behave relatively close to ideally for their pressure, volume, and temperature—but not for other properties. For example, a molecular gas such as oxygen (O2) obeys the ideal gas law fairly well at ordinary temperatures and pressures even though the gas is not composed of single, pointlike particles. The specific heat of oxygen and other molecular gases, however, differs substantially from the predictions of the ideal gas law. 
|
| (23.8) | | | P | = | pressure (Pa or N/m2) | | V | = | volume (m3) | | N | = | number of particles (molecules) | | kB | = | Boltzmann’s constant = 1.38×10-23 J/K | | T | = | absolute temperature (K) |
| Ideal gas law
for particles |
|
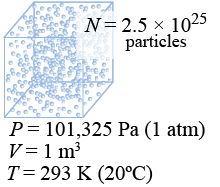 The quantity N in the ideal gas law is the number of particles. This is typically a very large number! At atmospheric pressure of 101,325 Pa, a cubic meter of air at room temperature of 293 K contains 2.5×1025 or 25 trillion trillion molecules!
The quantity N in the ideal gas law is the number of particles. This is typically a very large number! At atmospheric pressure of 101,325 Pa, a cubic meter of air at room temperature of 293 K contains 2.5×1025 or 25 trillion trillion molecules!

|
The number of particles is so large that equation (23.8) is inconvenient to work with, even though the equation accurately relates particle motion to temperature and pressure. Equation (23.9) is more useful because it is stated in terms of moles of gas n. The number of moles is calculated using Avogadro’s number: n = N/NA. Boltzmann’s constant is replaced in equation (23.9) by the ideal gas constant, which has the value R = 8.31 J/(mol K). 
|
| (23.9) | | | P | = | pressure (Pa or N/m2) | | V | = | volume (m3) | | n | = | number of moles of gas | | R | = | gas constant = 8.31 J/(K mol) | | T | = | absolute temperature (K) |
| Ideal gas law
for moles |
|
A pump compresses a volume of gas as shown in the diagram. What is the final temperature of the gas if the gas starts at 20ºC (293 K)? | Asked: | final temperature T2 | | Given: | P1, P2, V1, V2, T1 [at right] | | Relationships: | PV = nRT | | Solution: | The quantity of gas stays the same: | | Answer: | 318 K or 45ºC | | 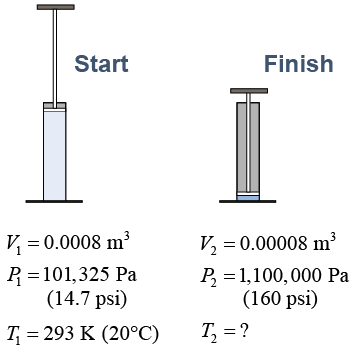 |

|
| |
|

