|
 Using very clever experiments, in 1895 French physicist Jean Perrin determined that there were about 6×1023 atoms in 1 g of hydrogen, the lightest element. Subsequent experiments have refined Avogadro’s number to its modern value of NA = 6.022×1023. The number is named in honor of the Italian scientist and teacher Amadeo Avogadro. One mole is defined as Avogadro’s number. For example, one mole of hydrogen atoms is 6.022×1023 atoms. The mole is one of the seven fundamental quantities of the SI system.
Using very clever experiments, in 1895 French physicist Jean Perrin determined that there were about 6×1023 atoms in 1 g of hydrogen, the lightest element. Subsequent experiments have refined Avogadro’s number to its modern value of NA = 6.022×1023. The number is named in honor of the Italian scientist and teacher Amadeo Avogadro. One mole is defined as Avogadro’s number. For example, one mole of hydrogen atoms is 6.022×1023 atoms. The mole is one of the seven fundamental quantities of the SI system. 
 |
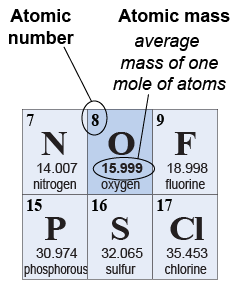 All the matter around us is made from 92 naturally occurring elements. The periodic table lists all the elements in order of increasing atomic number. Hydrogen has an atomic number of one, helium two, lithium three, and so on. Atoms of each different element have different masses; the periodic table lists the average atomic mass for each element. This is not the mass of one atom! Instead, the atomic mass is the mass of one mole of atoms. For example, the atomic mass of oxygen is listed as 15.999 g. That means that one mole of oxygen atoms, or 6.022×1023 atoms, has a combined mass of 15.999 g.
All the matter around us is made from 92 naturally occurring elements. The periodic table lists all the elements in order of increasing atomic number. Hydrogen has an atomic number of one, helium two, lithium three, and so on. Atoms of each different element have different masses; the periodic table lists the average atomic mass for each element. This is not the mass of one atom! Instead, the atomic mass is the mass of one mole of atoms. For example, the atomic mass of oxygen is listed as 15.999 g. That means that one mole of oxygen atoms, or 6.022×1023 atoms, has a combined mass of 15.999 g. 
|
| (23.4) | | | NA | = | number of atoms in one mole |
| Avogadro’s
number
|
|
You use statistics to calculate an average of a set of numbers, such as the average rent for an apartment in a certain city. The kinetic theory of matter uses statistics to analyze the average behavior of trillions of individual atoms. Kinetic theory shows how the average behavior of 1023 particles produces observable properties such as temperature and pressure. A fundamental result of kinetic theory is that the average energy of a single atom resulting from random thermal motion in any particular direction is given by equation (23.5), where kB is Boltzmann’s constant and T is the absolute temperature in kelvins. Equation (23.5) relates the microscopic property of an atom’s thermal energy U to its macroscopic property of temperature T. 
|
| (23.5) | | | E | = | energy (J) | | kB | = | Boltzmann’s constant = 1.38×10−23 J/K | | T | = | absolute temperature (K) |
| Thermal energy
per atom
|
|
If we did not have the historical measure of temperature in “degrees,” then we might instead use equation (23.5) to define temperature in units of energy. A temperature of 20ºC (293 K) is equivalent to 6.07×10−21 joules per particle, for example. Think of Boltzmann’s constant and Avogradro’s number as numerical “bridges” between the microscopic world of atoms and molecules and macroscopic properties such as density, temperature, and pressure. Boltzmann’s constant has a very small value at 1.38×10−23 J/K, which indicates that individual atoms have very small energies. 
 |
Equation (23.5) relates temperature to the average thermal energy per particle in each degree of freedom. A single atom that is free to move in the x-, y-, and z-directions has three degrees of freedom, one for each independent direction of motion. A gas of molecules in which each gas particle consists of two or more atoms has more degrees of freedom. For example, an oxygen molecule, consisting of two atoms of oxygen, can also rotate and stretch; each of these motions counts as another degree of freedom. 
|
One mole of carbon has a mass of 12 g. What is the mass of a single atom? | Asked: | mass of a single atom | | Given: | 12 g = 1 mole of carbon atoms | | Relationships: | 1 mole = 6.022×1023 particles | | Solution: | | | Answer: | 1.99×10−23 g/atom | 
|

