|
The Fahrenheit and Celsius scales are commonly used to measure temperature. Both are based on the properties of water. The Celsius scale defines zero degrees as the freezing point of water and 100 degrees as the boiling point of water. The Fahrenheit scale defines 32 degrees as the freezing point of water and 212 degrees as the boiling point of water. 
|
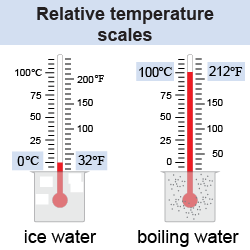 The Fahrenheit scale has 180 degrees between the boiling and freezing points of water. The Celsius scale, however, has 100 degrees between the same two points. Therefore, a change of 180°F is equivalent to a change of 100°C. To convert from degrees Celsius to degrees Fahrenheit, you multiply by a factor of 9/5 then add 32°, because 0ºC is the same as 32ºF. Equations (23.1) and (23.2) are used to convert temperatures between the two scales.
The Fahrenheit scale has 180 degrees between the boiling and freezing points of water. The Celsius scale, however, has 100 degrees between the same two points. Therefore, a change of 180°F is equivalent to a change of 100°C. To convert from degrees Celsius to degrees Fahrenheit, you multiply by a factor of 9/5 then add 32°, because 0ºC is the same as 32ºF. Equations (23.1) and (23.2) are used to convert temperatures between the two scales. 
 |
Let’s say that you want to convert a temperature from degrees Celsius into degrees Fahrenheit but that the only equation you remember—or the equation provided in a written exam—converts from degrees Fahrenheit into degrees Celsius. What do you do? Use algebra to derive the equation you need!
Starting with the equation you are given multiply both sides by 9/5 and then cancel out terms in the numerator and denominator: | |
Now add 32 to both sides: | |
and you have the equation you need: In a physics exam, you may sometimes be required to use algebra to manipulate a given equation into another one to calculate the answer. 
|
| (23.1) | | | TC | = | temperature in degrees Celsius (°C) | | TF | = | temperature in degrees Fahrenheit (°F) |
| Celsius
temperature scale |
| (23.2) | | | TF | = | temperature in degrees Fahrenheit (°F) | | TC | = | temperature in degrees Celsius (°C) |
| Fahrenheit
temperature scale |
|
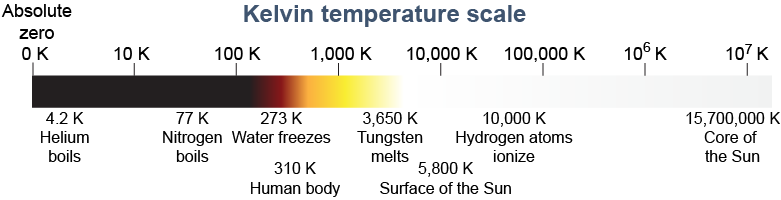
The choice of 0ºC as the freezing point of water is quite arbitrary in that water molecules do not have zero kinetic energy at 0ºC. Instead, the kinetic energy in molecular motion is effectively zero at a much lower temperature called absolute zero. Absolute zero is −273.15ºC (−459.7°F) and is the minimum possible temperature that matter can have. Temperature cannot go lower than absolute zero. 
 |
The Kelvin temperature scale is an absolute scale, such that there can be no temperature below absolute zero. Despite this firm statement, researchers have recently demonstrated that they can reach temperatures slightly below absolute zero using quantum effects in a gas. By using rapidly switching magnetic fields, the researchers caused the supercooled atoms to flip suddenly from their lowest energy state into a higher energy state—and then used lasers to keep the atoms in this state, which turned the gas into a slightly negative temperature. Aside from quantum physics “tricks” like this, however, you should rest assured that absolute zero is the lowest possible temperature in normal matter! 
|
|
The Kelvin scale of temperature starts at absolute zero. There are no “degrees” in the Kelvin scale, even though 1 K represents the same temperature difference as 1ºC. Since temperatures in kelvins are referenced to absolute zero, a temperature on the Kelvin scale is called an absolute temperature. Most equations relating to heat, such as the gas laws, use absolute temperatures in kelvins. You can convert between degrees Celsius and kelvins by using equation (23.3). 
|
| (23.3) | | | TK | = | temperature in kelvins (K) | | TC | = | temperature in degrees Celsius (°C) |
| Kelvin
temperature scale |
|
Which temperature is hotter, 200°F or 95°C?
 |
200°F is 93°C, so 95°C is hotter. 
|

