| | Essential questions | | What is the relationship among pressure, volume, and temperature for a gas? | |
|
In this simulation you will explore the behavior of ideal gases under different values of pressure, temperature, and volume. The simulation will change volume, temperature, and pressure according to the quantity and type of gas and the conditions you specify. 
|
Properties of various ideal gases
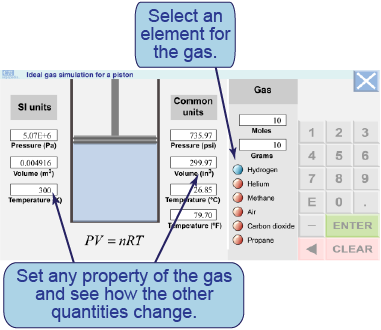
- Enter values in any of the text boxes to run the simulation.
- The temperature, volume, and pressure are limited by realistic properties of materials.
- What volume does 1 mol of air occupy at 101,325 Pa and 0ºC (273 K)? Compare this to the volume of other gases at the same conditions.
- Tire air pressure changes when temperatures change. Suppose a tire is inflated to a gauge pressure of 32 psi (absolute pressure of 46.7 psi) at 20ºC. What is the pressure if the temperature drops to −20ºC?
- If the same tire were to be driven on a road across the Sahara Desert, how would the pressure change? Estimate its gauge pressure and justify your value.
- What happens to the pressure when you double the quantity of gas while keeping volume and temperature constant?
- How much does the pressure change when you increase the volume by a factor of 10 while keeping temperature constant?
- If the temperature doubles, by how much does the volume have to change if pressure is held constant?
- What is the volume of 1 g of helium at atmospheric pressure and 0ºC?
- What is the volume of 1 g of carbon dioxide at atmospheric pressure and 0ºC?
- A propane tank for a barbeque contains 9,000 g of propane (20 lb). What volume does this occupy as a gas? Compare this to the actual volume of the propane tank. What do you conclude?
- At room temperature and for the same volume and gas mass, which of the gases in the simulation (hydrogen, helium, methane, air, carbon dioxide, or propane) has the highest pressure? Why do you think that is the case?

|
|
In this interactive simulation, you will see how the pressure, temperature, volume, and number (or mass) of gas particles are related to one another for the gas inside a sealed cylinder. This allows you to explore the properties of the gas inside a piston.
|
| |
|

