|
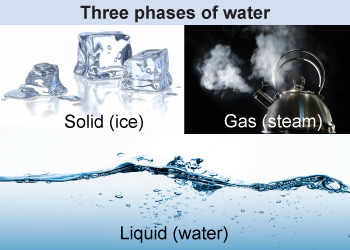 Matter can be a solid, liquid, or gas—three of the phases of matter—depending on its temperature. In the kinetic theory, the phase of matter for a particular substance is related to the average thermal energy of its atoms or molecules. At any given temperature, the phase of a material depends on the strength of the attractive forces that pull atoms and molecules together compared to the chaotic agitation of thermal motion.
Matter can be a solid, liquid, or gas—three of the phases of matter—depending on its temperature. In the kinetic theory, the phase of matter for a particular substance is related to the average thermal energy of its atoms or molecules. At any given temperature, the phase of a material depends on the strength of the attractive forces that pull atoms and molecules together compared to the chaotic agitation of thermal motion. 
 |
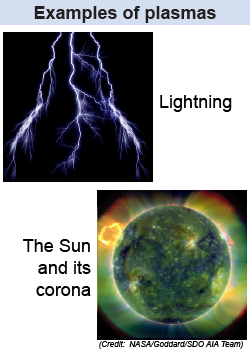 We learn from childhood about the three phases of matter: solid, liquid, and gas. But did you know that there’s a fourth? It’s called a plasma, which occurs when a gas is at a temperature high enough that some (or all) of the electrons break free from their atoms or molecules. Speeding around in a plasma are both (negatively charged) free electrons and the rest of the atoms (the positively-charged ions). Plasma is a good conductor of electricity, can block some wavelengths of light, and, because it is composed of charged particles, can be manipulated by both electric and magnetic fields.
We learn from childhood about the three phases of matter: solid, liquid, and gas. But did you know that there’s a fourth? It’s called a plasma, which occurs when a gas is at a temperature high enough that some (or all) of the electrons break free from their atoms or molecules. Speeding around in a plasma are both (negatively charged) free electrons and the rest of the atoms (the positively-charged ions). Plasma is a good conductor of electricity, can block some wavelengths of light, and, because it is composed of charged particles, can be manipulated by both electric and magnetic fields.
It takes a temperature of approximately 10,000 K to break electrons free from a hydrogen atom; even higher temperatures are required for many other atoms. Because of the high temperatures required, we don’t regularly come in contact with a plasma. There is, however, a physical phenomenon we see periodically that contains a plasma: lightning. The high energies of the electric current in lightning ionize the air around it—i.e., break electrons loose from the air molecules—which briefly turns that air into a plasma. The interior of the Sun, as well as the corona surrounding it, are also so hot that they contain plasma.
A solar flare is a highly energetic eruption of plasma from the surface of the Sun. The Solar Dynamics Observatory, a satellite launched by NASA, captured this video (button at left) of a solar flare on March 30, 2010. The size of the flare loop is 30 times the diameter of the Earth. 
|
 In a gas the thermal motion of atoms completely overcomes attractive forces between molecules. Molecules in a gas are typically far apart from each other. Water becomes a gas above its boiling point of 100ºC. Of the three phases, gas has the highest kinetic energy per molecule and occurs at the highest temperature.
In a gas the thermal motion of atoms completely overcomes attractive forces between molecules. Molecules in a gas are typically far apart from each other. Water becomes a gas above its boiling point of 100ºC. Of the three phases, gas has the highest kinetic energy per molecule and occurs at the highest temperature. 
|
 Between 0ºC and 100ºC water is a liquid. Molecules in a liquid have enough thermal energy to break away temporarily from their neighbors and change places with one another, but liquid molecules do not have enough thermal energy to separate completely and become a gas. Liquids flow because molecules can change places with their neighbors.
Between 0ºC and 100ºC water is a liquid. Molecules in a liquid have enough thermal energy to break away temporarily from their neighbors and change places with one another, but liquid molecules do not have enough thermal energy to separate completely and become a gas. Liquids flow because molecules can change places with their neighbors. 
 |
Intramolecular covalent bonds between the hydrogen and oxygen atoms in a water molecule are nearly 25 times stronger than the intermolecular bonds between two different water molecules. The intermolecular forces between liquid or solid particles are called van der Waals forces, named after the Dutch physicist Johannes van der Waals who discovered them and explained their major properties. These covalent bonds form a negative potential energy among the atoms in a molecule; it takes extra energy to break those bonds and separate the atoms from each other in the molecule. Molecules in a liquid far more easily break their bonds between molecules—bonds resulting from the van der Waals forces—than do molecules in a solid, because the solid molecules have much stronger bonds holding the molecules themselves together. That is why each individual molecule in a liquid holds together even though it is repeatedly breaking and reforming bonds with its neighbors. 
|
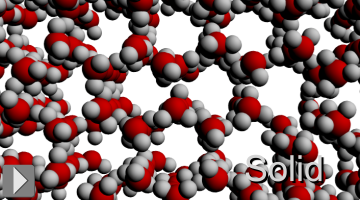 Below 0ºC water is a solid. Matter becomes solid when thermal energy is too low to overcome intermolecular forces. Molecules in a solid are still vibrating, but an average molecule does not have enough energy to break away from its immediate neighbors or to exchange places with another molecule. That is why solids hold their shape.
Below 0ºC water is a solid. Matter becomes solid when thermal energy is too low to overcome intermolecular forces. Molecules in a solid are still vibrating, but an average molecule does not have enough energy to break away from its immediate neighbors or to exchange places with another molecule. That is why solids hold their shape. 
|
The temperature at which a substance changes phase depends on the relative strength of attractive forces between molecules compared to the thermal energy. Metals have strong intermolecular forces, so they are solid at room temperature. Substances with weak intermolecular forces, such as argon, are a gas at room temperature. The melting point is the temperature at which a substance transitions between solid and liquid; the boiling point is the temperature at which it transitions between liquid and gas. 
|
|
It takes extra energy to be absorbed by a liquid for it to evaporate into a gas, whereas energy is released for the gas to condense back into a liquid. Similarly, energy is absorbed by a solid to melt it into a liquid and released to freeze the liquid back into a solid.
|
Which common phase of matter has the weakest intermolecular forces? - solid
- liquid
- gas
 |
The correct answer is c, gas. In a gas, the molecules are moving so fast that they break the bonds between each other and go flying off. 
|

