|
In the first section of this chapter, we described how matter is made of particles—atoms and molecules—that are in constant thermal motion. The ideas of specific heat and thermal energy were mostly described at the macroscopic level that you experience in daily life. In this section, we dig into the microscopic physics of matter on the molecular scale. Starting with ideal gases we will see how properties such as specific heat are derived from the motions and interactions of atoms. In any ordinary quantity of matter, such as the gas in a balloon, there are so many atoms that their average behavior must be studied statistically. The kinetic theory uses statistical techniques to relate the macroscopic properties of matter to the physics of matter on the microscopic scale. 
|
Statistics and macroscopic properties
|
Atoms are so small that we do not experience individual particles. Instead, we experience the average behavior of trillions and trillions of particles. Statistics is the branch of mathematics that deals with the properties of large groups of numbers. When you calculate an average you are using statistics. Statistical mechanics is the branch of physics that explains how the average behavior of trillions of microscopic particles creates properties such as temperature, density, and pressure. 
|
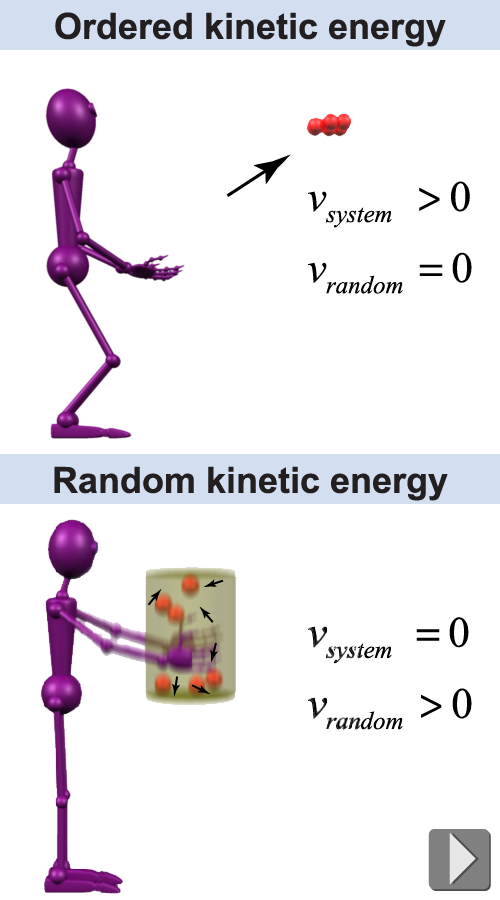 The kinetic energy of a single particle is ½mv2, similar to the kinetic energy of a baseball. A whole collection of particles can actually have two kinds of kinetic energy: ordered and random. To appreciate the difference, consider a handful of ping-pong balls. If you throw the whole handful, the group of balls has ordered kinetic energy because there is an average velocity. But suppose you put them in a jar and then vigorously shake the jar up and down. The balls will bounce madly around as individuals but the average velocity of the whole group together is zero. Random motion—such as for the ping-pong balls in a jar—has an average velocity of zero, but the average speed is not zero. That is why the bouncing balls inside the jar have nonzero kinetic energy. Normal matter may have 1023 atoms bumping into each other a trillion times a second. The constant bumping creates exchanges of energy and momentum between particles, and this keeps the motion truly random.
The kinetic energy of a single particle is ½mv2, similar to the kinetic energy of a baseball. A whole collection of particles can actually have two kinds of kinetic energy: ordered and random. To appreciate the difference, consider a handful of ping-pong balls. If you throw the whole handful, the group of balls has ordered kinetic energy because there is an average velocity. But suppose you put them in a jar and then vigorously shake the jar up and down. The balls will bounce madly around as individuals but the average velocity of the whole group together is zero. Random motion—such as for the ping-pong balls in a jar—has an average velocity of zero, but the average speed is not zero. That is why the bouncing balls inside the jar have nonzero kinetic energy. Normal matter may have 1023 atoms bumping into each other a trillion times a second. The constant bumping creates exchanges of energy and momentum between particles, and this keeps the motion truly random. 
|
Avogadro’s number is such an enormously large value than only statistical methods can deal with atoms in any observable quantity of matter. The kinetic theory of matter applies the principles and tools of statistical mechanics to create a comprehensive explanation for how matter behaves. For example, temperature is the average kinetic energy of molecules resulting from random motion. Boltzmann’s formula for the average thermal energy per degree of freedom of a single atom (E = ½kBT) comes from kinetic theory. Kinetic theory provides the fundamental explanation for virtually all the properties of matter we observe, including pressure, specific heat, electrical conductivity, and viscosity. 
|
|
|
| |
|

