|
A fluid is incompressible when its volume stays roughly constant despite changes in pressure. Most liquids are incompressible. A fluid is compressible when it changes volume proportional to a change in pressure. Gases are compressible. A gas will expand its volume to fill any size container, usually changing both pressure and temperature in the process. 
|
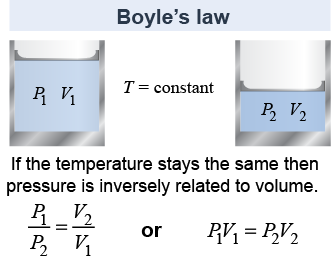 Imagine a volume of gas confined in a cylinder by a sliding piston. As the piston moves up and down, the amount of gas (mass) stays the same, but the volume, pressure, and temperature can change. If the volume is decreased then the pressure increases. This is mathematically stated by Boyle's law, which says that the change in pressure is inversely proportional to the change in volume, or P ∝ 1/V, when temperature is held constant. The converse is also true: If the volume goes up, then the pressure goes down.
Imagine a volume of gas confined in a cylinder by a sliding piston. As the piston moves up and down, the amount of gas (mass) stays the same, but the volume, pressure, and temperature can change. If the volume is decreased then the pressure increases. This is mathematically stated by Boyle's law, which says that the change in pressure is inversely proportional to the change in volume, or P ∝ 1/V, when temperature is held constant. The converse is also true: If the volume goes up, then the pressure goes down.
Boyle’s law is often written in the form P1V1 = P2V2, where P1V1 are the initial pressure and volume and P2V2 are the final values. With this form, the final pressure and volume are inversely proportional as P2 ∝ 1/V2. 
|
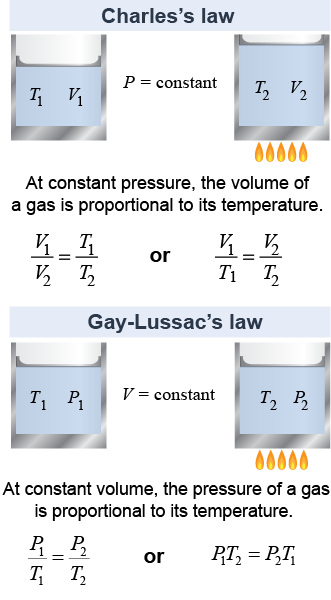
When pressure is kept constant, gases expand and contract proportional to their absolute temperature, or V ∝ T. This rule is known as Charles’s law after French scientist and balloonist Jacques Charles.
If instead the volume is kept fixed, then the pressure changes proportional to the change in absolute temperature, or P ∝ T. This last rule is known as Gay-Lussac’s law after another French scientist, Joseph Gay-Lussac, who discovered it experimentally in 1809. 
| 
|
The temperature and pressure that appear in these equations are absolute values. For temperature that means T must be in kelvins, which are referenced to absolute zero. To convert to kelvins add 273 to the Celsius temperature. For example, 10ºC is 283 K. The absolute pressure must include the pressure of the atmosphere if appropriate. An ordinary tire pressure gauge reads pressure above atmospheric. A gauge pressure of 100,000 Pa therefore represents an absolute pressure of 201,325 Pa after including 101,325 Pa for the atmosphere. 
|
| |
|

