|
For thousands of years humans have wondered about the nature of electricity, light, and heat. Aristotle thought fire was an element, like water, air, and earth, and that its natural place was in the Sun, which explained why smoke rises. After a few thousand years of ideas, observations, and especially experiments, we now have an explanation that correctly fits all the observed facts. Electricity, light, and heat have their explanation in the microscopic structure of both matter and energy. 
|
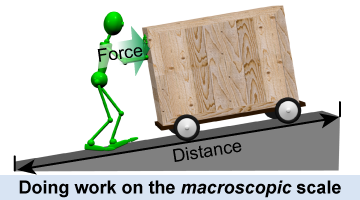 Physicists use the concept of scale to describe the relative sizes of things. When you apply a force to push a cart up a ramp you can see the transfer of energy as the cart moves uphill. This is an example of work being done on the macroscopic scale. The macroscopic scale refers to things we can touch and sense directly. Rocks, shopping carts, dust specks, and planets are macroscopic objects. The macroscopic scale is the scale of ordinary life, from about 1/100th of a millimeter and larger.
Physicists use the concept of scale to describe the relative sizes of things. When you apply a force to push a cart up a ramp you can see the transfer of energy as the cart moves uphill. This is an example of work being done on the macroscopic scale. The macroscopic scale refers to things we can touch and sense directly. Rocks, shopping carts, dust specks, and planets are macroscopic objects. The macroscopic scale is the scale of ordinary life, from about 1/100th of a millimeter and larger. 
|
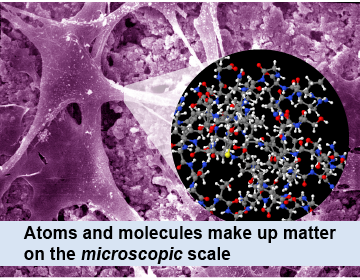 Hidden inside the macroscopic world is the microscopic world of atoms and particles. In physics, the microscopic scale refers to the size of an atom and smaller. Atoms are so small that even a dust speck contains trillions of atoms. A single atom has a diameter of around 10−10 m. In the context of physics, the word microscopic refers to things much, much smaller than are visible with an ordinary optical microscope.
Hidden inside the macroscopic world is the microscopic world of atoms and particles. In physics, the microscopic scale refers to the size of an atom and smaller. Atoms are so small that even a dust speck contains trillions of atoms. A single atom has a diameter of around 10−10 m. In the context of physics, the word microscopic refers to things much, much smaller than are visible with an ordinary optical microscope. 
|

|
Electricity, heat, and light can only be fully understood by considering the behavior of matter and energy on the microscopic scale. For example, electrons, the tiny particles responsible for most observable aspects of electricity, typically move distances of 10−9 m between collisions with other electrons. Photons, the basic units of light, have energies of about 3×10−19 joules. Temperature is a measure of the average kinetic energy of individual atoms (or molecules) moving around vigorously, even in solid matter. 
|
In physics, are very small organisms, such as bacteria, on the macroscopic scale or the microscopic scale?
 |
They are on the macroscopic scale. Although a microscope is needed to view them, physics includes bacteria on the macroscopic scale because they are still much larger than atoms and follow the physics of the macroscopic scale, not atomic physics. 
|
| |
|

