|
A very fundamental way to think of force is as the active agent through which energy moves. Energy is constantly being transformed from one form to another in every second of our existence. When work is done by a force, that work transfers energy from one object to another or transforms the energy from one form to another. Work is the means through which energy is transformed into and out of mechanical forms, such as potential and kinetic energy. 
|
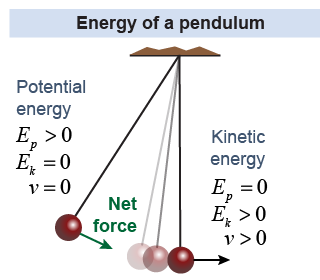 Consider a pendulum swinging back and forth. The pendulum has both kinetic energy and potential energy. At different points in its motion the energy is all of one form or the other, but the balance continually shifts. When the swinging mass is at the highest point in each swing, the energy is all potential. When the mass is lowest (and moving the fastest) the energy is all kinetic. The energy moves back and forth through the action of the net force, which is a component of the gravitational force.
Consider a pendulum swinging back and forth. The pendulum has both kinetic energy and potential energy. At different points in its motion the energy is all of one form or the other, but the balance continually shifts. When the swinging mass is at the highest point in each swing, the energy is all potential. When the mass is lowest (and moving the fastest) the energy is all kinetic. The energy moves back and forth through the action of the net force, which is a component of the gravitational force. 
|
In a closed system, if one kind of energy increases in value, then one or more other kinds of energy must decrease by the same amount to keep the total energy constant. Another way to express the law of energy conservation is by using energy changes: The total change in energy for a closed system must equal zero. 
|
| (10.3) | | | ΔE | = | change in total energy of system (J) |
| Energy conservation
alternative formula |
|
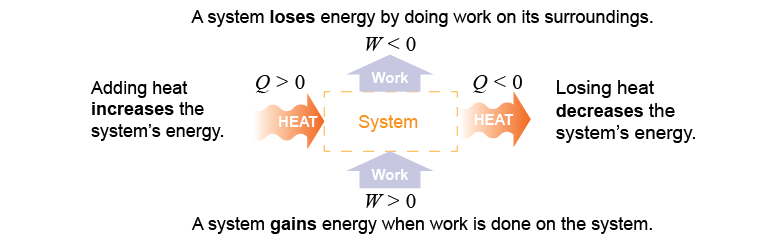
For an open system, the energy equation is written as equation (10.4) below, for which ΔW is the work done on the system. Equation (10.4) says that the change in total energy of a system equals the heat added Q plus the work done on the system. Many systems convert some energy into heat through friction. On the next page we will see that many energy technologies, such as a car engine, convert heat into work. This general version of the law of conservation of energy extends the work–energy theorem and is known as the first law of thermodynamics. We will learn more about it on page 730 in Chapter 25. 
|
| (10.4) | | | ΔE | = | change in energy (J) | | Q | = | heat added (J) | | W | = | work done on the system (J) |
| First law of
thermodynamics
|
|
|
Malinda is at the top of a mountain and is just beginning her descent on skis. As she accelerates downward, her gravitational potential energy is - increasing.
- decreasing.
- unchanged.
- zero.
 |
The answer is b, decreasing. As she goes down the mountain, her elevation above sea level decreases, so her gravitational potential energy also decreases. 
|

