|
The total energy in a closed system remains constant. Any change in the system that increases one form of energy, such as kinetic energy, can only do so if another form of energy decreases by the same amount. This is a powerful way of looking at changes. Changes involve the exchange of energy. Any change that increases a system’s energy in one form can only occur in parallel with one or more changes that decrease the system’s energy in other forms. 
|

|
The law of conservation of energy applies to the total energy of a system, and not to any one form of energy. Some or all of the energy can be transformed from one form to another, as long as the total energy remains constant. “Constant” means that the total energy in a closed system at any time is the same as the system started with. 
 |
There are a number of conservation laws in physics, not just the conservation of energy, that you will encounter throughout this book: (linear) momentum, angular momentum, and electric charge. The total number of electric charges, for example, is constant in time; charge cannot be created or destroyed in a closed system.
When doing physics at the atomic level, there are two other important conservation laws governing elementary particles: the number of baryons (such as neutrons and protons) and the number of leptons (such as electrons, muons, and neutrinos). Don’t commit that last one to memory yet, though: Recent scientific results on neutrino oscillations appear to violate the lepton number conservation law and may point the way to exciting new physics!
Most of the time we can also think of mass as being conserved. But Albert Einstein put forward a model using his famous equation E = mc2, in which mass–energy (not mass) is conserved. In Einstein’s equation, mass can be converted into energy and vice versa, so you cannot consider the total mass of a closed system without considering the total energy, too. Einstein’s equation becomes important in atomic physics and for physics at very high speeds (approaching the speed of light). 
|
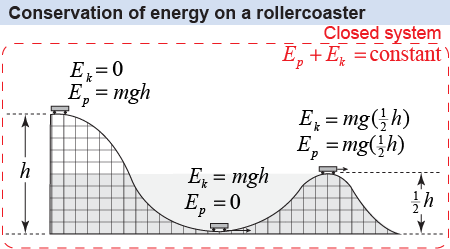 To see how this is useful for understanding how things change, consider a car on a roller coaster. Let the system be the roller coaster and car, including the effect of gravity. If we assume the system is closed, then the energy at the start is all potential because the car is at rest on top of the hill.
To see how this is useful for understanding how things change, consider a car on a roller coaster. Let the system be the roller coaster and car, including the effect of gravity. If we assume the system is closed, then the energy at the start is all potential because the car is at rest on top of the hill. 
|
At some later time the car is rolling fast at the very bottom of the hill. How fast can the car go? Energy conservation tells us that if potential energy and kinetic energy are the only forms of energy in the system, then the car’s kinetic energy gain can only come from potential energy lost. The kinetic energy at h = 0 must equal the lost potential energy. Therefore, we can set ½mv2 equal to mgh and solve for the speed at the bottom of the hill. Since the mass cancels from both sides, conservation of energy tells us that the speed of the car is completely determined by the change in height h. 
|
| (10.1) | | | Einitial | = | initial total energy (J) | | Efinal | = | final total energy (J) |
| Conservation of energy
|
|
Equation (10.1) is a good way to apply the law of conservation of energy to solving physics problems. In a closed system the total energy at the start is the same as the total energy at any later time or in any other configuration of the system. 
|
In physics, the term “conservation” means something different from what it does in conversation. When we talk about “conserving” energy in everyday life, we mean to use less energy. When you leave a room, you turn off the lights to “conserve” energy. When a truck is loading, it will turn off its engine to “conserve” fuel. When people speak of “running out of energy” what they are really referring to is that certain forms of energy, such as fossil fuels, are limited resources. “Using” these resources does not destroy the energy; instead, the energy is turned into heat, chemical change, and other forms. 
 |
An electrical battery by itself can be thought of as a closed system that starts with chemical energy and converts it into electrical energy. When you connect the battery to a circuit, the battery by itself is no longer a closed system; the battery plus circuit becomes a new closed system with constant energy. The Sun starts with nuclear energy and converts it into thermal energy that heats up its interior. As long as a system is closed, energy is conserved inside it—no matter what form (or forms) that the energy is transformed into. 
|
|
|
If a closed system has 500 J of energy right now, how much will it have in five minutes? - 0 J
- 500 J
- 1,000 J
- There is not enough information to determine the answer.
 |
Because this is a closed system, the law of conservation of energy applies and energy cannot enter or leave. Therefore, the system will always have 500 J of energy. 
|

