|
 Imagine dropping a beach ball from the top of a building. At first, its measured speed would agree very well with the theoretical formula we derived from energy conservation, but at some point it cannot fall any faster. It’s not that energy conservation is wrong; it’s that we neglected to account for air friction slowing down the beach ball’s descent. The real speed of the beach ball is slower than the theoretical speed because of friction.
Imagine dropping a beach ball from the top of a building. At first, its measured speed would agree very well with the theoretical formula we derived from energy conservation, but at some point it cannot fall any faster. It’s not that energy conservation is wrong; it’s that we neglected to account for air friction slowing down the beach ball’s descent. The real speed of the beach ball is slower than the theoretical speed because of friction. 
|
The efficiency of a process describes how well the process transforms input energy into output energy. When you drop a ball, the input energy is the ball’s potential energy, while the output energy is its kinetic energy. 
|
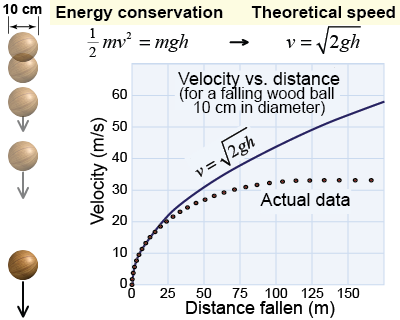 If you drop a wood ball, friction causes the efficiency to vary with time. At speeds up to 10 m/s, the process of falling is close to 100% efficient—all of the potential energy lost by dropping in height is converted into kinetic energy. At speeds above 10 m/s, however, the efficiency gradually diminishes as more and more of the potential energy is spent overcoming air friction, while less is left to become kinetic energy. This is shown in the graphical model at right, where you can see the actual speed of the ball is increasingly different from the model in which no friction is assumed. At the terminal velocity of 33 m/s, the efficiency becomes zero, because all of the potential energy is lost overcoming friction and none of it is available to become kinetic energy.
If you drop a wood ball, friction causes the efficiency to vary with time. At speeds up to 10 m/s, the process of falling is close to 100% efficient—all of the potential energy lost by dropping in height is converted into kinetic energy. At speeds above 10 m/s, however, the efficiency gradually diminishes as more and more of the potential energy is spent overcoming air friction, while less is left to become kinetic energy. This is shown in the graphical model at right, where you can see the actual speed of the ball is increasingly different from the model in which no friction is assumed. At the terminal velocity of 33 m/s, the efficiency becomes zero, because all of the potential energy is lost overcoming friction and none of it is available to become kinetic energy. 
|
| (10.5) | | | η | = | efficiency | | Eout | = | output energy of a system (J) | | Ein | = | input energy of a system (J) |
| Efficiency
|
|
Efficiency is the ratio of the output energy of a system to its input energy. Since work and energy are directly related, the definition of efficiency can also be expressed as the ratio of the work performed by a system to the work input to the system. If a man does 2,000 J of work pushing a box up a ramp, and the box gains 1,200 J of potential energy, then this process is only 60% efficient because the man spent 40% of his work overcoming friction. 
|
Efficiency is a very important parameter in technology. The best solar cells commercially available convert only 15% of the power in sunlight into electrical power. Typical rechargeable batteries are 20% efficient, which means that you get only 20 J of output energy for every 100 J of input energy you spend charging the battery. A solar power system that can provide electricity overnight requires both solar cells and batteries. Together, the system efficiency is 15% × 20% or only 3%! That means as much as 97% of the original solar energy ends up as waste heat. Many scientists and engineers are working to improve the efficiency of both solar cells and batteries. 
 |
 We can use some simple numbers to estimate the power production of the Hoover Dam. It has an average hydraulic head height of 160 m, the effective vertical distance that water from its reservoir drops to power its turbines. The gravitational potential energy of a liter of water (which has a mass of 1 kg) is therefore
We can use some simple numbers to estimate the power production of the Hoover Dam. It has an average hydraulic head height of 160 m, the effective vertical distance that water from its reservoir drops to power its turbines. The gravitational potential energy of a liter of water (which has a mass of 1 kg) is therefore | |
With rare exceptions, the entire 500,000 L/s flow rate of the Colorado River flows through the dam’s turbines—not over its spillway (the overflow route that accommodates times of heavy rains and flooding). The efficiency of a hydroelectric turbine can reach 0.80 (or 80%), so an average power production for the dam is approximately | |
Our estimate of a power generating capacity of 630 MW is not far off from the long-term average quoted by the U.S. Department of the Interior of 480 MW. In any case, that is a lot of power! 
|
If Max does 500 J of work spinning a 10% efficient generator, how much electrical energy will he produce?
 |
Eout = Ein × η, so 500 J × 10% = 50 J of electrical energy. 
|
| |
|

