|
Up to now, we have considered the energy within a system before and after a change. But energy can also enter or leave an open system. Mechanical energy enters or leaves a system through work, the action of forces. Work done on a system increases the system’s total energy, whereas work done by a system decreases its energy. This section connects work done with the conservation of energy. 
|
Work and energy
|
As you learned on page 255, work is a form of energy. The work done by a force is the force multiplied by the distance moved in the direction of the force. Consider a system containing an uncompressed spring at its free length. A force acts from outside the system to compress the spring a distance x. The final energy of the system is the initial energy it started with plus the work done on the system by the external force. 
|
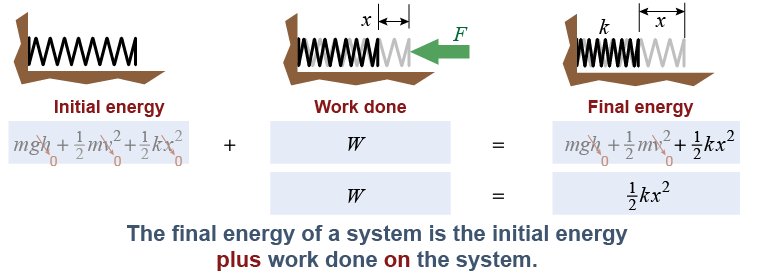
|
Again, consider the system to consist only of the spring. If the spring is now used to launch a ball, then the system does work on something outside the system: the ball. When a system exerts a force that does work outside the system, then the final energy is the initial energy minus the work done by the system. 
|
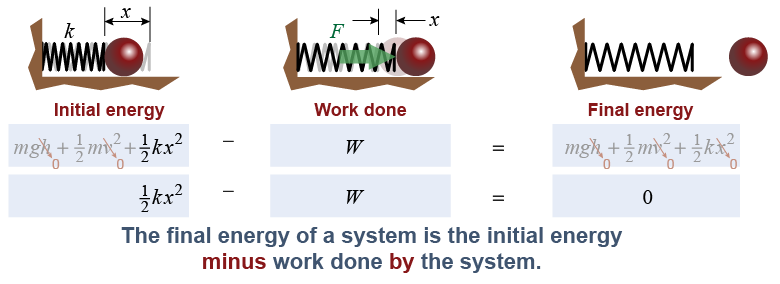
|
If all forces act inside the system, then the total energy of the system remains constant because all the energy lost by one part of the system is gained by another part. If forces act outside the system, then the energy of the system either increases when work is done on the system or decreases when the system does work on the outside environment. Notice how the initial and final energies are not equal for the spring system illustrated above because there are forces acting outside the system. 
|

