|
One of the foundational laws of physics is that the total amount of energy in a closed system does not change. As events happen, such as a ball rolling downhill, energy changes from one form to another. Nonetheless, the total energy remains constant. Any process that requires a certain amount of energy will not occur if there is insufficient energy available. This section addresses the law of conservation of energy and how this law governs all processes in nature. 
|
Systems and interactions
|
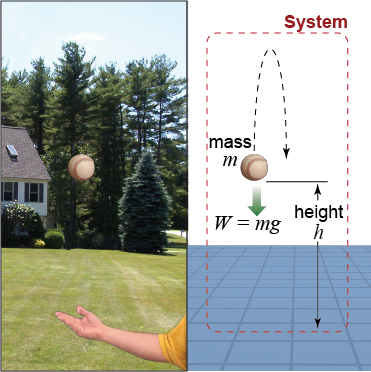 If we want to understand the speed of a falling ball, we might include the ball’s initial height, mass, and speed, as well as the force of gravity. The color of the sky or exact materials inside the ball are information we exclude because these details do not matter to what we are trying to understand. A group of interacting objects and influences is called a system. We choose a system to include only the objects and influences—such as forces—that are important to what we are investigating. Choosing a system frees us from having to consider everything, which would be an impossible task.
If we want to understand the speed of a falling ball, we might include the ball’s initial height, mass, and speed, as well as the force of gravity. The color of the sky or exact materials inside the ball are information we exclude because these details do not matter to what we are trying to understand. A group of interacting objects and influences is called a system. We choose a system to include only the objects and influences—such as forces—that are important to what we are investigating. Choosing a system frees us from having to consider everything, which would be an impossible task. 
|
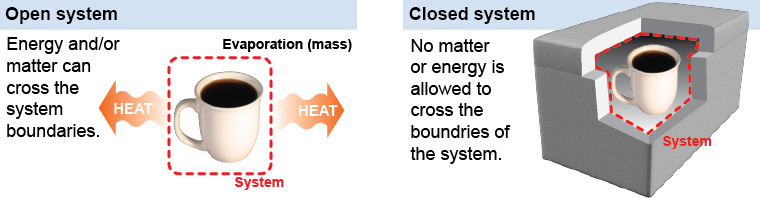
A system can be thought of as everything within an imaginary shell, or system boundary, that completely encloses the behavior you want to understand. If we allow matter or energy to cross the boundary then we say the system is open. A cup of hot coffee on a table is an open system. In a few hours the thermal energy leaks out into the room and in a few days the water evaporates away, leaving only the coffee residue in the bottom of the cup. Both matter and energy have left the system. If no matter or energy or force can cross the boundary then we say the system is closed. Closed systems are useful to think about, because in a closed system the total energy remains constant. The total energy at one time will be the same as the total energy at some later time. In an open system, however, the energy can change because the surroundings can add or remove energy from it. 
|
|
While there is no such thing as a truly closed system, it is often a very good approximation to assume a closed system as a starting point for an investigation. The analysis is much easier and we get the “big picture” of what happens. We can start with a simplified model and then add details based on comparing the model’s predictions with actual data. 
 |
In many experimental situations, such as a car rolling down a ramp, the leakage of energy into forms not counted—such as thermal energy (or heat)—is small compared to the matter and energy that are included in the system. In these cases it may be a very good approximation to treat the system as if it were perfectly closed. Even if the energy leakage is substantial, the closed-system approximation provides a useful reference for identifying important missing details such as friction. Scientists almost always start with a simplified model and then add details based on comparing the predictions of the model with the actual behavior from experiments or observations. 
|
Is the Earth a closed system?
 |
No. Energy enters the Earth from the Sun and some mass can enter when asteroids hit the Earth. 
|

