| | Essential questions | | How is color related to the frequency and wavelength of light?
How are changes in frequency related to changes in wavelength? | |
|
What physical property or properties of light make red light different from blue or green light? In this short interactive simulation, you will compare a visible spectrum of light—ranging from violet to red light—with the frequencies and wavelengths of the light. 
|
Part 1: Relating the color of light to its frequency and wavelength
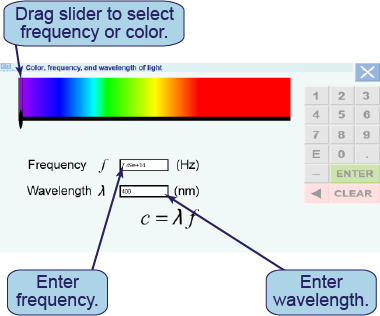 In the interactive simulation, there is a horizontal spectrum of light ranging from violet to red. You can change the light in any of three different ways:
In the interactive simulation, there is a horizontal spectrum of light ranging from violet to red. You can change the light in any of three different ways: - by moving the slider from left to right;
- by entering a value in the box for the frequency (e.g., using “6.0 e14” to represent 6.0×1014 Hz); or
- by entering a value in the box for the wavelength (in nanometers).
- What is the wavelength of blue light? Cyan? Green? Record the wavelengths using scientific notation and correct SI units.
- What color of light corresponds to a wavelength of λ = 580 nm?
- What color of light corresponds to a frequency of f = 6.9×1014 Hz?
- What color(s) of the visible spectrum have the highest frequencies? Longest wavelengths?
- Put the following colors in order of increasing frequency: blue, green, indigo, orange, red, violet, and yellow. Can you think of a mnemonic to remember this order of the colors?
- How are frequency and wavelength related to each other? In other words, if you change the light to have a longer wavelength, how does the frequency of the light change?

|
|
In this interactive simulation, you will investigate how the color of visible light is related to its frequency and wavelength. Drag the slider to different colors of light, or enter a frequency or wavelength, to explore their interrelationships.
|
Part 2: Compare to RGB color combinations

- On page 22, you looked for the best RGB match for each of the colors on the right.
- Now using the interactive simulation of the visible light spectrum, look for the best match in the visible light spectrum for each of the colors on the right.
- Tabulate the wavelengths you found and describe whether you consider the color to be a good match.
- For which colors could you make a good match with the visible light spectrum?
- For which colors could you find no suitable match? Explain why this is the case.

|
| |
|

