|
| Essential questions | | How does the photoelectric effect depend on the frequency and intensity of
the incident light?
Why does it require a quantum physics explanation? | |
|
Nineteenth-century physicists uncovered a most peculiar interaction between light and matter that they called the photoelectric effect. Light shining on a metal will liberate electrons, creating electric current. Lower frequency light, however, does not liberate electrons—no matter how bright the source of the light. Why would a faint but blue light source create electric current while an intense red light source would not? In this investigation you will recreate the photoelectric effect in an interactive simulation. 
|
Part 1: Threshold frequency
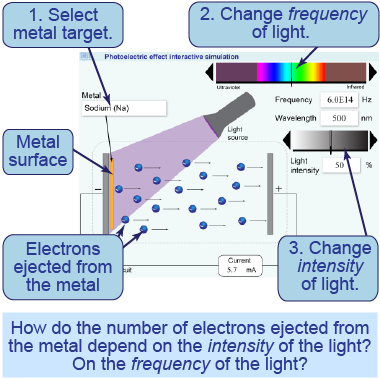
- Launch the interactive simulation. Select calcium.
- Set the light color to violet using the frequency slider.
- Increase the intensity of the light source to around 50% so that electrons are ejected from the metal surface.
- Change the light to different wavelengths—such as red light or ultraviolet—and observe the changes to the ejected electrons.
- Repeat this test for three other metals.
- Does UV light eject electrons? Green light? Red?
- What is the threshold frequency? Are electrons ejected at lower or higher frequencies than this threshold?
- Above the threshold frequency, how does the electron speed change with frequency?
- Above the threshold frequency, how does electric current change with frequency? Collect and tabulate current data every 5 s for 30 s for two different frequencies to support your answer.
- Research the work function, threshold frequency, and/or threshold wavelength for the metals that you analyze, such as by looking them up in the
CRC Handbook of Chemistry and Physics. Do your values agree with the experimentally measured values? 
|
|
In this interactive simulation, you will investigate the photoelectric effect. You can change the intensity of the incident light, its frequency, and the kind of metal it is shining upon. See what properties of the light cause electrons to be ejected from the metal.
|
Part 2: Intensity of the light
- Set the frequency above the threshold. Observe the ejected electrons as you vary the light intensity.
- Set the frequency below the threshold. Observe the ejected electrons as you vary the light intensity.
- How does the number of ejected electrons vary with light intensity above the threshold frequency?
- How does the number of ejected electrons vary with light intensity below the threshold frequency?
- Above the threshold frequency, how does the electron speed change with the light intensity?
- Why does the photoelectric effect require a quantum explanation? In your answer, refer to the threshold frequency and the effect of changing the intensity of the light.

|

