|
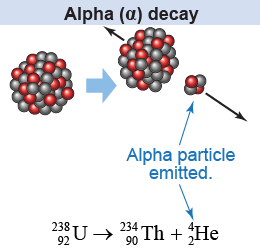 An alpha particle is the nucleus of a helium atom, consisting of two protons and two neutrons. Since the alpha particle has no electrons, it has a net positive charge, which makes it different from an ordinary, neutral helium atom. When alpha decay occurs, the nucleus emits an alpha particle that carries a modest amount of kinetic energy away with it. The energy of an alpha particle is low enough to be blocked by just a piece of paper!
An alpha particle is the nucleus of a helium atom, consisting of two protons and two neutrons. Since the alpha particle has no electrons, it has a net positive charge, which makes it different from an ordinary, neutral helium atom. When alpha decay occurs, the nucleus emits an alpha particle that carries a modest amount of kinetic energy away with it. The energy of an alpha particle is low enough to be blocked by just a piece of paper! 
 |
Remember the Rutherford scattering experiment? In that experiment, alpha particles were shot at a target of gold foil. They were deflected from the target gold nuclei by the electric force of repulsion, because both the alpha particle and the target nucleus have positive charge. Now you know more about the significance of the alpha particle because of its connection with radioactivity and alpha decay! 
|
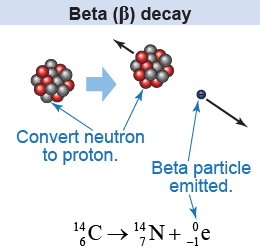 A neutron may spontaneously transform into a proton and an electron: . This type of transformation is done via a process called beta decay (β decay). When a nucleus undergoes beta decay, it emits an energetic electron or a positron. These energetic particles are called beta particles—denoted as β− or β+. Beta particle is a historical term for an electron. Originally, it was Ernest Rutherford who coined the term beta radiation to distinguish it from the alpha radiation that he also discovered and used to study the nucleus.
A neutron may spontaneously transform into a proton and an electron: . This type of transformation is done via a process called beta decay (β decay). When a nucleus undergoes beta decay, it emits an energetic electron or a positron. These energetic particles are called beta particles—denoted as β− or β+. Beta particle is a historical term for an electron. Originally, it was Ernest Rutherford who coined the term beta radiation to distinguish it from the alpha radiation that he also discovered and used to study the nucleus. 
|
Similarly, a proton may transform into a neutron and a positively charged particle called a positron: . The positron is denoted by or β+ and it is a positively charged electron. 
|
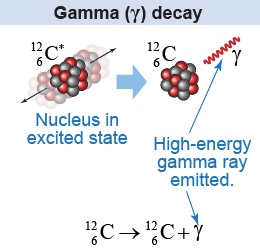 In Chapter 26 we learned that quantum theory forces electrons confined to an atom to have energy levels. The same is true of nucleons confined to the nucleus, except that the energies are much higher because they involve the strong nuclear force. A nucleus is in an excited state when a proton or neutron occupies an energy level above the nuclear ground state. An excited nucleus releases energy by emitting a high-energy photon or γ ray via the process of gamma decay (γ decay).
In Chapter 26 we learned that quantum theory forces electrons confined to an atom to have energy levels. The same is true of nucleons confined to the nucleus, except that the energies are much higher because they involve the strong nuclear force. A nucleus is in an excited state when a proton or neutron occupies an energy level above the nuclear ground state. An excited nucleus releases energy by emitting a high-energy photon or γ ray via the process of gamma decay (γ decay). 
|
Gamma decay is almost always the result of leftover energy from another decay or nuclear reaction. When a nucleus undergoes alpha or beta decay, some of the change in binding energy appears as kinetic energy of the emitted particle. Part of the remaining energy puts the nucleus in an excited state. The excited nucleus goes to a lower energy state by releasing a γ ray. 
|
Gamma decay is analogous to the emission of visible light by an atom as an electron transitions from a higher to a lower energy level. The energy of a γ-ray photon, however, is much higher than the energy of a visible photon. Gamma rays are at the high-energy and high-frequency region of the electromagnetic spectrum. Gamma rays are very penetrating and to stop them requires material with high atomic mass and high density, such as lead. 
|
If uranium-238 undergoes alpha decay, what is the product? - thorium-234
- protactinium-234
- uranium-234
- neptunium-234
 |
The answer is a, thorium-234. When uranium-238 undergoes alpha decay, it loses two protons, so its atomic number decreases from 92 to 90. Atomic element 90 is thorium. Its mass number also decreases by four, going from 238 to 234. 
|

